仿生动态纳米药物SrHCF:基于Fe2+反应的晶格自重构实现“铁螯合-抗氧化-钙拮抗-炎症抑制”四联治疗CIRI
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
血运重建术已经彻底改变了缺血性卒中(IS)的治疗,但其疗效受到脑缺血再灌注损伤(CIRI)的限制。CIRI涉及多层病理机制的动态相互作用,如铁凋亡、钙超载、氧化应激和随后的炎症细胞因子风暴,这使得许多新兴的单靶点治疗策略在缓解CIRI方面受到限制。同时协同靶向CIRI多个关键病理因素的策略仍然备受期待,但极具挑战性。本研究构建了一种类似sr -取代普鲁士蓝(PB)的纳米药物(SrHCF),作为治疗CIRI的最终多功能纳米平台。具体来说,SrHCF首先具有高活性的伪超氧化物歧化酶(SOD)/过氧化氢酶(CAT)酶,可以有效地消除活性氧(ROS)。其次,SrHCF的Fe3+-CN-Sr2+配位网络有效捕获Fe2+并触发晶格重构,将其转化为具有更强抗氧化活性的PB;第三,同步释放Sr2+,有效拮抗Ca2+。由于这种多途径的治疗协调机制,SrHCF可以同时抑制神经元铁下沉,减少氧化应激和防止钙超载。这些协同作用使SrHCF能够保护线粒体,减轻内质网应激,最终显著减少小胶质细胞cGAS-STING通路激活引起的神经元死亡和炎症风暴。本研究为CIRI治疗提供了一个有希望的多靶点协同调控范式。本文章由计算机程序翻译,如有差异,请以英文原文为准。
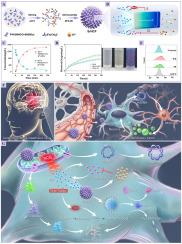
Biomimetic dynamic nanomedicine SrHCF: Lattice self-reconstruction based on Fe2+ response to achieve synergistic “ferrous chelation-antioxidation-calcium antagonism-inflammation inhibition” quadruple treatment of CIRI
Revascularization has revolutionized the treatment of ischemic stroke (IS), but its efficacy is limited by cerebral ischemia-reperfusion injury (CIRI). CIRI involves the dynamic interaction of multidimensional pathological mechanisms such as ferroptosis, calcium overload, oxidative stress, and subsequent inflammatory cytokine storm, which make many emerging single-target therapeutic strategies limited in alleviating CIRI. Strategies that simultaneously and synergistically target multiple key pathological factors in CIRI remain highly anticipated but extremely challenging. This study constructed a Sr-substituted Prussian blue (PB)-like nanodrug (SrHCF) as the ultimate multifunctional nanoplatform for the treatment of CIRI. Specifically, SrHCF firstly holds highly active pseudo-superoxide dismutase (SOD)/catalase (CAT) enzymes to effectively eliminate reactive oxygen species (ROS). Secondly, the Fe3+-CN-Sr2+ coordination network of SrHCF efficiently captures Fe2+ and triggers lattice reconstruction to convert it into PB with stronger antioxidant activity and then thirdly, synchronously releases Sr2+ to effectively antagonize Ca2+. Due to this multi-pathway therapeutic coordination mechanism, SrHCF can simultaneously inhibit neuronal ferroptosis, reduce oxidative stress and prevent calcium overload. These synergistic effects enable SrHCF to protect mitochondria and alleviate endoplasmic reticulum stress, ultimately significantly reducing neuronal death and the inflammatory storm caused by the activation of the cGAS-STING pathway of microglia. This study provides a promising paradigm of multi-target synergistic regulation for CIRI treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


