四味西藏毛如汤通过肠道微生物依赖的SCFA修复和免疫调节减轻胶原诱导的关节炎
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
类风湿性关节炎(RA)是由肠道微生物群失调及其代谢物失调影响的免疫失衡引起的。目的通过肠道微生物群-代谢物-免疫轴,探讨SXD缓解类风湿关节炎的机制。方法建立胶原诱导关节炎大鼠模型。采用16S rRNA测序和代谢组学技术评估SXD对肠道菌群和短链脂肪酸代谢的影响。流式细胞术定量测定血液和脾脏中免疫细胞的比例。此外,我们利用Caco-2细胞来评估SXD和丁酸盐对amp活化蛋白激酶(AMPK)信号激活的影响。结果ssxd可显著缓解关节炎症状,降低血清TNF-α和IL-17水平。SXD逆转了厚壁菌门/拟杆菌门的比例,抑制了致病属Desulfovibrio,并丰富了产生scfa的Butyricicoccus。SXD显著上调ZO-1、occludin和下调zonulin,同时促进丁酸盐等SCFAs的生物合成。SXD和丁酸盐通过AMPK途径增强Caco-2细胞紧密连接蛋白的表达。相关分析显示,SCFA水平与紧密连接蛋白/抗炎因子呈显著正相关,与促炎因子、肠通透性标志物、Th1/Th2、Th17/Treg呈显著负相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
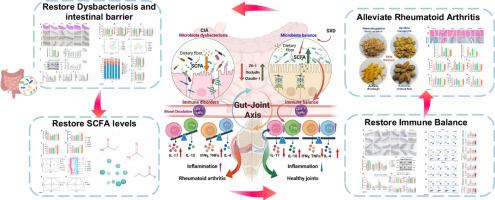
Siweixizangmaoru decoction attenuates collagen-induced arthritis via gut microbiota-dependent SCFA restoration and immunomodulation
Background
Rheumatoid arthritis (RA) arises from immune imbalance that may be affected by gut microbiota dysbiosis and dysregulation of their metabolites. Siweixizangmaoru decoction (SXD), a classical formula in Traditional Tibetan Medicine (TTM), alleviates RA symptoms, yet its mechanisms remain unclear.
Purpose
The aim of this research is to elucidate the mechanism by which the SXD alleviates RA through the gut microbiota-metabolite-immune axis.
Methods
A collagen-induced arthritis rat model was established. The effects of SXD on gut microbiota and short-chain fatty acid (SCFA) metabolism were assessed using 16S rRNA sequencing and metabolomics. Flow cytometry performed to quantify the proportions of immune cell in blood and spleen. Moreover, Caco-2 cells was utilized to assess the effects of SXD and butyrate on AMP-activated protein kinase (AMPK) signaling activation.
Results
SXD significantly alleviated arthritis symptoms and decreased serum TNF-α and IL-17 levels. SXD reversed the Firmicutes/Bacteroidetes ratio, suppressing the pathogenic genus Desulfovibrio and enriching SCFA-producing Butyricicoccus. SXD significantly upregulated ZO-1, occludin and downregulated zonulin, while enhancing biosynthesis of SCFAs like butyrate. SXD and butyrate enhance tight junction protein expression in Caco-2 cells via AMPK pathway. Correlation analysis revealed strong positive correlations between SCFA levels and tight junction proteins/anti-inflammatory factors, while negative correlations were observed with pro-inflammatory cytokines, intestinal permeability markers and the Th1/Th2 and Th17/Treg ratio.
Conclusion
This study demonstrates that SXD alleviates RA by synergistically regulating the gut microbiota-SCFA metabolism-immune homeostasis axis, providing a promising approach for Tibetan medicine targeting the "gut-joint axis" in RA therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


