坡缕石与铜铝层状双氢氧化物复合材料在废水中硝酸盐污染物去除中的应用
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-04-16
DOI:10.1016/j.jiec.2025.04.032
引用次数: 0
摘要
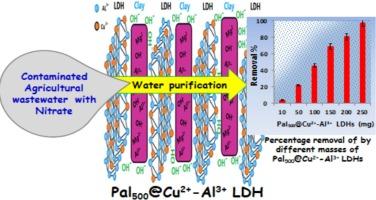
硝酸盐对环境的污染是当今人们关注的主要问题。由于硝酸盐肥料的极端使用,硝酸盐形成高度稳定的含氮化合物。然而,大多数水处理方法在去除硝酸盐和产生二次废物方面都是无效的。因此,本研究旨在组装一种新型、可持续和环保的Palygorskite (Fullers土)-固定化cu2 +/Al3+ LDHs (Pal500@Cu2+-Al3+ LDHs)复合材料。对10.0 ~ 20.0 mg L-1 NO3 -的去除率进行了深入研究,得出pH 3.0、时间50 min、温度25℃、复合材料10 mg为最佳条件。Pal500@Cu2+- al3 + LDHs对NO3 -的去除采用伪一阶模型和Bangham表达式相结合的方法。此外,热力学参数证实了NO3 -通过放热和自发吸附过程去除,包括解离机制。通过离子对相互作用和阴离子交换机制,提出了NO3 -离子与Pal500@Cu2+- al3 +的相互作用和结合模式。此外,200和250 mg Pal500@Cu2+- al3 + LDHs对实际农业排水废水的NO3 -回收率分别为81.0和98.0%。此外,所探索的Pal500@Cu2+-Al3+ LDHs在吸附和解吸再生过程中具有良好的稳定性,长达5个连续循环。因此,本研究收集的结果验证了Pal500@Cu2+- al3 + LDHs对实际农业排水废水中NO3 -的特殊去除的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Implementation of integrated Palygorskite with copper–aluminum layered double hydroxides composite in superior nitrate pollutant removal from wastewater
Environmental pollution with nitrate is a major issue of concern these days. Nitrate form highly stable nitrogenous compounds due to the extreme usage of nitrate fertilizers. However, most water treatment methods are ineffective in terms of nitrate removal and generation of secondary wastes as well. Consequently, this investigation is directed to assemble a novel, sustainable and ecofriendly Palygorskite (Fullers earth)-immobilized-Cu2+/Al3+ LDHs (Pal500@Cu2+-Al3+ LDHs) composite. Removal of 10.0 - 20.0 mg L-1 NO3– were thoroughly investigated and significantly concluded that pH 3.0, time 50 min, temperature 25 °C and 10 mg composite are the optimum conditions. The removal of NO3– by Pal500@Cu2+-Al3+ LDHs was accomplished by a combination of pseudo-first-order model and Bangham expressions. Additionally thermodynamic parameters confirmed NO3– removal via exothermic and spontaneous adsorption process including a dissociative mechanism. The interaction and binding modes of NO3– ions to Pal500@Cu2+-Al3+ were proposed via ion-pair interaction and anion-exchange mechanisms. Additionally, 200 and 250 mg of Pal500@Cu2+-Al3+ LDHs exhibited excellent NO3– recovery from real agricultural drainage wastewater providing 81.0 and 98.0 %, respectively. Moreover, the explored Pal500@Cu2+-Al3+ LDHs afforded good stability towards adsorption and desorption regeneration processes up to five successive cycles. Consequently, the collected results from this study verify the validity of Pal500@Cu2+-Al3+ LDHs for exceptional removal of NO3– from real agricultural drainage wastewater.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


