果蝇神经元细胞内乳酸动力学
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
乳酸产生和消耗的速率反映了包括神经元在内的许多细胞类型的代谢状态。在这里,我们研究了营养剥夺对果蝇神经元乳酸动力学的影响,利用未搅拌层的限制作用,这是一种在培养细胞周围形成的扩散屏障。我们发现,当海藻糖(昆虫喜欢的一种葡萄糖双糖)的可用性受到未搅拌层的限制时,神经元会组成性地消耗乳酸。未搅拌层的急性机械破坏减少了对乳酸盐的依赖。通过动力学建模和实验验证,我们证明了未搅拌层条件下神经元乳酸消耗率与细胞线粒体密度成反比。此外,神经元乳酸水平表现出时间相关性,这使得摄动后的乳酸动态可以通过摄动前的值来预测。总的来说,我们的研究结果揭示了自然形成的扩散屏障对神经元代谢偏好的影响,并证明了营养剥夺条件下乳酸动力学与随后营养可用性恢复所引起的乳酸动力学之间存在时间相关性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
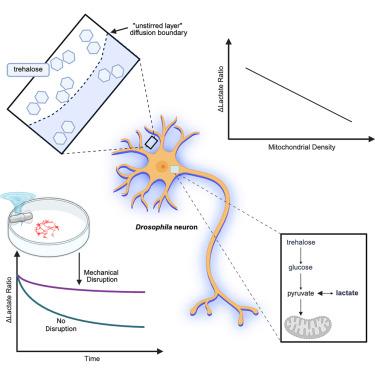
Intracellular lactate dynamics in Drosophila neurons
Rates of lactate production and consumption reflect the metabolic state of many cell types, including neurons. Here, we investigated the effects of nutrient deprivation on lactate dynamics in Drosophila neurons by leveraging the limiting effects of the unstirred layer, a diffusion barrier that forms around cells in culture. We found that neurons constitutively consume lactate when the availability of trehalose, a glucose disaccharide preferred by insects, is limited by the unstirred layer. Acute mechanical disruption of the unstirred layer reduced this reliance on lactate. Through kinetic modeling and experimental validation, we demonstrate that neuronal lactate consumption rates under unstirred layer conditions correlate inversely with the cells’ mitochondrial density. Furthermore, neuronal lactate levels exhibited temporal correlations that allowed postperturbation lactate dynamics to be predicted by preperturbation values. Collectively, our findings reveal the influence of naturally forming diffusion barriers on neuronal metabolic preferences and demonstrate the existence of temporal correlations between lactate dynamics under conditions of nutrient deprivation and those evoked by the subsequent restoration of nutrient availability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


