少突胶质细胞祖细胞的分形因子依赖性小胶质细胞吞噬调节小脑白质发育
IF 4.1
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
神经发育障碍通常包括运动和认知障碍,与大脑和小脑区域的成熟有关。本研究表明,在出生后早期发育过程中,变形样小胶质细胞通过第四脑室区浸润小脑白质,与大脑白质的类似活动平行。这些小胶质细胞在过渡到分支状态之前,在有限的时间窗口内吞噬少突胶质细胞祖细胞。裂肽受体信号的破坏(已知介导突触修剪)减少了小胶质OPC的吞噬,导致少突胶质细胞的增加和髓鞘发育的改变。fractalkine受体基因的变异与神经发育障碍有关,如自闭症和精神分裂症,这两种疾病都涉及髓磷脂异常。这些发现强调了变形虫小胶质细胞在形成大脑和小脑白质发育中的协调作用,表明这一过程的破坏可能导致神经发育障碍患者的脑回路和功能改变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
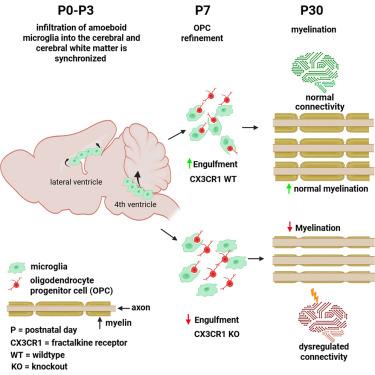
Cerebellar white matter development is regulated by fractalkine-dependent microglia phagocytosis of oligodendrocyte progenitor cells
Neurodevelopmental disorders often involve both motor and cognitive impairments, linked to the maturation of cerebral and cerebellar regions. This study reveals that during early postnatal development, ameboid microglia infiltrate the cerebellar white matter via the fourth ventricular zone, in parallel with similar activity in the cerebral white matter. These microglia phagocytose oligodendrocyte progenitor cells (OPCs) within a restricted time window before transitioning to a ramified state. The disruption of fractalkine receptor signaling—known to mediate synaptic pruning—reduces microglial OPC engulfment, leading to an increase in oligodendrocytes and altered myelin development. Variants in the fractalkine receptor gene have been linked to neurodevelopmental disorders such as autism and schizophrenia, both of which involve myelin abnormalities. These findings highlight a coordinated role of ameboid microglia in shaping white matter development in both the cerebrum and cerebellum, suggesting that disruptions in this process may contribute to altered brain circuitry and function in neurodevelopmental disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


