柠檬酸交联鼠尾草水凝胶吸收Cd(II)的批量和柱式模型研究:Box-Behnken设计优化
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-04-27
DOI:10.1016/j.jiec.2025.04.046
引用次数: 0
摘要
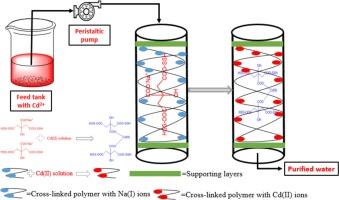
本研究从Salvia spinosa (SSH)种子中分离得到多糖基水凝胶,以柠檬酸(CA)为交联剂,先酯化成柠檬酸交联Salvia spinosa水凝胶(CL-SSH),再与碳酸氢钠(NaHCO3)水溶液皂化,形成吸附剂柠檬酸交联Salvia spinosa水凝胶(Na-CL-SSH)的钠盐。利用傅里叶红外光谱(FTIR)、固态交叉极化魔角自旋核磁共振光谱(CP/MAS 13CNMR)和扫描电镜(SEM)对CL-SSH进行了表征。采用导电点零电荷pH (pHZPC)法测定了Na-CL-SSH的表面电荷。采用响应面法Box-Behnken设计(RSM-BBD)对Cd(II)吸附数据进行预优化。模型设计方差分析的决定系数(R2 = 0.9980)较高,f值(358.61)大于p值(< 0.0001),且非显著拟合缺失(LOF)表明二阶多项式方程对Cd(II)吸附数据的拟合性。吸附动力学、吸附热力学和再生研究表明,Cd(II)的吸附是一个快速的放热过程,是通过离子交换机制进行的。同时进行了吸附Cd(II)的柱吸附实验,所获得的数据可为Na-CL-SSH吸附Cd(II)工艺的工业规模化提供参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A model batch and column study for Cd(II) uptake using citric acid cross-linked Salvia spinosa hydrogel: Optimization through Box-Behnken design
Herein, a polysaccharide-based hydrogel from Salvia spinosa (SSH) seeds was isolated and first esterified to citric acid cross-linked Salvia spinosa hydrogel (CL-SSH) using citric acid (CA) as cross-linker and then saponified with aqueous solution of sodium bicarbonate (NaHCO3) to form adsorbent, i.e., sodium salt of citric acid cross-linked Salvia spinosa hydrogel (Na-CL-SSH). The CL-SSH was characterized using Fourier transform infrared spectroscopy (FTIR), solid-state cross-polarization magic angle spinning nuclear magnetic resonance spectroscopy (CP/MAS 13CNMR), and scanning electron microscopy (SEM) analyses. The surface charge on Na-CL-SSH was determined by conductionng point zero-charge pH (pHZPC) analysis. The adsorption conditions were pre-optimized by applying response surface methodology Box-Behnken design (RSM-BBD) on the Cd(II) adsorption data. The high value of coefficient of determination (R2 = 0.9980), greater F-value (358.61) than p-values (< 0.0001) for ANOVA of the model design, and non-significant lack of fit (LOF) were obtained that indicated the fitness of second-order polynomial equation on the Cd(II) adsorption data. The study of adsorption kinetics, adsorption thermodynamics, and regeneration revealed that the Cd(II) adsorption was a rapid and exothermic process and occurred through ion-exchange mechanism. The column adsorption experiments for Cd(II) adsorption were also conducted and obtained data from the continuous study could be useful for scalability of the Cd(II) adsorption process by Na-CL-SSH to an industrial scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


