二硫化钼基纳米复合材料除汞效率的比较研究
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-04-24
DOI:10.1016/j.jiec.2025.04.041
引用次数: 0
摘要
通过应用先进的吸附剂实现对汞污染水的有效和可逆的修复是非常有利的,但仍然是一项具有挑战性的技术努力。本研究对比研究了还原氧化石墨烯/二硫化钼(rGO/MoS2)和镁铝层状双氢氧化物/二硫化钼(Mg-Al LDH/MoS2)两种MoS2(二硫化钼)基纳米复合材料对汞的吸附能力。纳米复合材料通过简单的水热法合成,然后使用x射线衍射,傅里叶变换红外光谱,扫描电子显微镜和brunauer - emmet - teller表面积分析方法对其进行表征。为了确定汞的吸附效果,设计了实验,考察了吸附剂用量、pH值、离子强度、初始汞离子浓度和接触时间等因素对汞吸附效果的影响。等温线数据调整为Langmuir, Freundlich和Temkin。Hg2+在rGO/MoS2和Mg-Al MgAl Mg-Al LDH/MoS2上的吸附符合Langmuir模型,为单层吸附。结果表明,rGO/MoS2和Mg-Al LDH/MoS2均具有较高的吸附量,分别为544.5 mg.g - 1和282.9 mg.g - 1, rGO/MoS2由于其增大的表面积和与MoS2之间的协同作用而表现出优异的吸附性能。由范霍夫图得到的热力学参数证实了吸附过程的自发和吸热特性。Hg2+离子与纳米复合材料的相互作用途径包括Hg-S络合和静电吸引。吸附动力学符合准二级模型。分析物的扩散过程受到颗粒内扩散和膜内扩散机制的共同影响。两种纳米复合材料都表现出强大的可重复使用性,在至少5次循环中保持超过94%的吸附容量。通过评估其在真实水基质中的性能,包括从不同来源获得的去离子水、自来水、井水和河水,评估了制备的吸附剂在实际应用中的可行性。这项比较研究打开了使用这些纳米复合材料从污染水中高效去除Hg2+的可能性,为环境修复中先进材料的开发提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
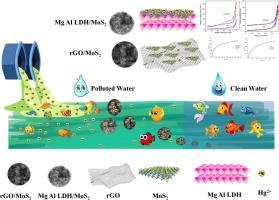
Comparative study on the efficiency of mercury removal from aqueous medium using MoS2-based nanocomposites
Achieving the efficient and reversible remediation of mercury-polluted water through the application of advanced sorbents is highly advantageous but remains a challenging technical endeavor. The study provides a comparative investigation into the adsorption capabilities of Hg using two MoS2 (molybdenum disulfide) based nanocomposites: reduced graphene oxide/molybdenum disulfide (rGO/MoS2) and magnesium–aluminum layered double hydroxide/molybdenum disulfide (Mg-Al LDH/MoS2). Synthesis of the nanocomposites was achieved via a simple hydrothermal approach, followed by their characterization using X-ray diffraction, Fourier-transform infrared spectroscopy, scanning electron microscopy, and Brunauer–Emmett–Teller surface area analysis methodologies. To determine the effectiveness of mercury adsorption, experiments were designed to examine the influence of several factors, including the amount of adsorbent, pH value, ionic strength, initial mercury ion concentration, and the duration of contact. The isotherm data were adjusted to the Langmuir, Freundlich and Temkin. The adsorption of Hg2+ onto the rGO/MoS2 and Mg-Al MgAl Mg-Al LDH/MoS2 was consistent with the Langmuir model, indicating monolayer adsorption. Results revealed that both rGO/MoS2 and Mg-Al LDH/MoS2 exhibited high adsorption capacities of 544.5 and 282.9 mg.g−1 respectively, rGO/MoS2 showcasing superior performance due to its enhanced surface area and synergistic effect between rGO and MoS2. The thermodynamic parameters derived from van’t Hoff plots confirm the spontaneous and endothermic characteristics of the adsorption process. The interaction pathway of Hg2+ ions with the nanocomposites involves Hg-S complexation alongside electrostatic attraction. The adsorption kinetics followed the pseudo-second order model. The process of analyte diffusion was jointly influenced by both intraparticle diffusion and film diffusion mechanisms. Both nanocomposites demonstrated robust reusability, retaining over 94 % adsorption capacity for at least 5 cycles. The feasibility of the as-prepared adsorbents for real-world applications was assessed by evaluating its performance in authentic water matrices, including deionized water, tap water, well water, and river water, obtained from diverse sources. This comparative study opens the possibility to use of these nanocomposites for efficient Hg2+ removal from polluted water, providing insights for the development of advanced materials in environmental remediation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


