CO2地质封存条件下多组分电解质溶液中CO2溶解度及地球化学反应的定量研究
IF 7.9
Q1 ENGINEERING, MULTIDISCIPLINARY
引用次数: 0
摘要
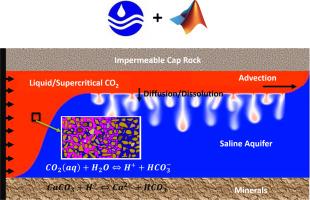
CO2溶解度捕获和CO2-盐水-矿物相互作用是了解CO2在地质储存过程中的命运和迁移行为的关键。开发了一种先进的仪器来精确量化CO2在盐水中的溶解度和CO2-盐水-矿物相互作用。尽管如此,实验测量可能是耗时和资源密集的。CO2SolTool是将MATLAB与Phreeqc集成开发的,它提供了一种可靠的方法来确定盐水中溶解的CO2的数量,饱和CO2盐水的性质(例如pH和密度),以及矿物、盐水和CO2之间的相互作用。计算结果与实验结果吻合较好。CO2SolTool可以在很宽的温度、压力、盐水盐度和成分范围内,准确模拟存在气体杂质的盐水中溶解二氧化碳的数量。Pitzer模型对于盐水盐度升高时的热力学和地球化学计算是足够精确的。H2S的溶解度比CO2高,而N2和CH4的溶解度比CO2小。电解质,尤其是HCO3−对饱和co2盐水的pH值有显著影响。H2S等杂质的存在可能会适度降低CO2饱和盐水的pH值,因为在相同条件下,H2S在盐水中的溶解度高于CO2。当存在白云石或方解石等矿物时,在二氧化碳-盐水矿物达到平衡后,pH值可能升高。硬石膏和石膏对饱和co2盐水pH值的影响有限。电势决定离子HCO3−、CO32−、Ca2+对方解石表面的电荷有很大的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Quantifying CO2 solubility and geochemistry reactions in multicomponent electrolyte solutions under CO2 geological sequestration conditions
CO2 solubility trapping and CO2-brine-mineral interactions are essential for understanding the fate and migration behavior of CO2 during geological storage. An advanced instrument was developed to accurately quantify the CO2 solubility in brine and CO2-brine-mineral interactions. Nonetheless, the experimental measurements can be time-consuming and resource-intensive. CO2SolTool, developed by integrating MATLAB with Phreeqc, provides a reliable approach for determining the quantity of dissolved CO2 in brine, the properties (e.g., pH and density) of CO2-saturated brine, and the interactions between mineral, brine, and CO2. The calculated data are consistent with the experimental findings. CO2SolTool can accurately simulate the quantity of dissolved CO2 in brine in the presence of gas impurities throughout a wide range of temperature, pressure, and brine salinity and compositions. The Pitzer model is sufficiently accurate for thermodynamic and geochemical calculations at elevated brine salinity. The solubility of H2S is higher than that of CO2, while the solubility of N2 and CH4 is smaller than that of CO2. The electrolytes, especially the HCO3−, have a significant effect on the pH value of CO2-saturated brine. The presence of impurities such as H2S may moderately reduce the pH level of the CO2-saturated brine, as it has higher solubility in brine than CO2 under the same conditions. When minerals, e.g., dolomite or calcite, are present, the pH levels may rise after the CO2-brine-mineral is equilibrated. Anhydrite and gypsum have limited impact on the pH value of CO2-saturated brine. The potential determining ions, including HCO3−, CO32−, Ca2+, can largely affect the electrical charge of the surface of calcite.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Results in Engineering
Engineering-Engineering (all)
CiteScore
5.80
自引率
34.00%
发文量
441
审稿时长
47 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


