Ba3Nb2O8六方钙钛矿衍生物氧化物的制备及输运性能
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
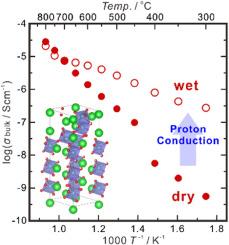
六方钙钛矿衍生物因其在高温下具有较高的氧化离子导电性而受到越来越多的关注。在这项工作中,选择原始的Ba3Nb2O8作为研究目标,因为这种成分是一些快速氧化物离子导体(如Ba3MoNbO8.5)的基石。因此,有关Ba3Nb2O8的制备和输运性质的知识对进一步设计优异的氧化物离子导体具有重要价值。研究了固相反应法制备Ba3Nb2O8单相的工艺参数,确定了最佳烧结温度为1200℃,烧结时间为36 ~ 48 h。暴露在潮湿大气中,Ba3Nb2O8可以水化以激活质子传导,这可以通过在潮湿大气中较高的体积电导率和观测到的H2O/D2O同位素来证明。通过电动势测量进一步确定了质子传导输运数在500℃时约为0.6,并随着温度的升高而减小。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Preparation and transport properties of Ba3Nb2O8 hexagonal perovskite-derivative oxide
Hexagonal perovskite-derivatives attract increasing attention due to their high oxide ion conductivity at high temperature. In this work, pristine Ba3Nb2O8 was chosen as the research target, since this composition is the cornerstone for some fast oxide ion conductors like Ba3MoNbO8.5. The knowledge on preparation and transport properties of Ba3Nb2O8 is thereby valuable to the further design of excellent oxide ion conductors. The parameters for the solid-state reaction method to prepare a Ba3Nb2O8 single phase were studied, and the optimal temperature and time for sintering were found to be 1200 °C and 36–48 h, respectively. By exposing to a humid atmosphere, Ba3Nb2O8 can be hydrated to activate proton conduction, which is evidenced by higher bulk conductivity in the wet atmosphere and observation of H2O/D2O isotope. The transport number of proton conduction is further determined by electromotive force measurements to be around 0.6 at 500 °C and decreases with the increasing temperature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


