铋基异质结的带界面协同工程用于声动力-化学动力协同治疗乳腺癌
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
超声免疫治疗的临床疗效受到超声增敏剂低活性氧(ROS)产量和肿瘤微环境(TME)内抗氧化防御机制的限制。本文利用带隙和界面工程策略,通过离子交换方法制备了铋基纳米异质结BiF3:Ce-BiOI-PEG (BCOP)。BCOP将高效的声催化ROS生成与tme响应的fenton样催化活性结合在一起,实现了声动力治疗(SDT)和化学动力治疗(CDT)的协同增强。在超声(US)照射下,BCOP异质结利用其内置电场驱动定向载流子分离,显著提高ROS的产生效率。同时,酸性TME触发Ce3+介导的fenton样反应,将内源性H2O2转化为高毒性羟基自由基(•OH)。此外,双谷胱甘肽(GSH)通过Bi3+配位和空穴(h+)介导的氧化有效地削弱了TME的抗氧化能力,协同放大了氧化应激诱导的肿瘤细胞损伤。体外细胞实验表明,BCOP可诱导乳腺癌细胞线粒体损伤、细胞凋亡和免疫原性细胞死亡(ICD)。体内研究进一步证实了其激活全身抗肿瘤免疫反应和显著抑制肿瘤生长的能力。本研究为多模式肿瘤免疫治疗提供了一种带-界面协同调控策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
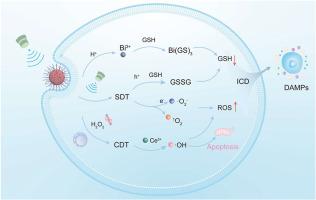
Band-interface cooperative engineering of bismuth-based heterojunctions for sonodynamic-chemodynamic synergistic breast cancer therapy
The clinical efficacy of sono-immunotherapy is limited by the low reactive oxygen species (ROS) yield of sonosensitizers and the antioxidant defense mechanisms within the tumor microenvironment (TME). Herein, leveraging bandgap and interfacial engineering strategies, we fabricate a bismuth-based nanoheterojunction BiF3:Ce-BiOI-PEG (BCOP) via an ion-exchange method. BCOP integrates efficient sono-catalytic ROS generation with TME-responsive Fenton-like catalytic activity, enabling synergistic enhancement of sonodynamic therapy (SDT) and chemodynamic therapy (CDT). Under ultrasound (US) irradiation, the BCOP heterojunction significantly boosts ROS production efficiency by utilizing its built-in electric field to drive directional carrier separation. Concurrently, the acidic TME triggers a Ce3+-mediated Fenton-like reaction, converting endogenous H2O2 into highly toxic hydroxyl radicals (•OH). Furthermore, dual glutathione (GSH) depletion via Bi3+ coordination coupled with hole (h+)-mediated oxidation effectively impairs the antioxidant capacity of the TME, synergistically amplifying oxidative stress-induced damage in tumor cells. In vitro cell experiments demonstrate that BCOP induces mitochondrial damage, apoptosis, and immunogenic cell death (ICD) in breast cancer cells. In vivo studies further confirm its ability to activate a systemic anti-tumor immune response and markedly inhibit tumor growth. This study provides a band-interfacial cooperative regulation strategy for multimodal tumor immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


