巨噬细胞相关免疫应答对聚醚酮酮骨植入物:单细胞转录组分析
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
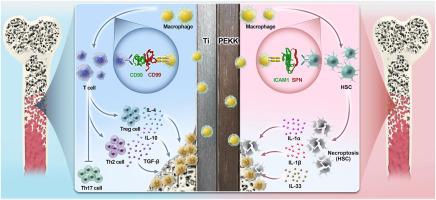
聚醚酮酮(PEKK)已成为钛(Ti)骨植入物的潜在替代品。然而,其骨整合性能不如钛,主要是由于对其早期免疫反应的了解有限。为了解决这一局限性,本研究利用单细胞RNA测序来研究基于ti和基于pek的植入物引发的不同的早期巨噬细胞反应。这种方法能够表征植入后骨髓微环境中的巨噬细胞极化动力学和细胞间相互作用。研究结果揭示了巨噬细胞表型的材料依赖性二元分化:Ti植入物优先招募Cd99+巨噬细胞,建立促进骨整合的抗炎微环境。相反,PEKK植入物募集Icam1+巨噬细胞,导致持续炎症和造血干细胞(hsc)应激。此外,Ti表面促进巨噬细胞和T细胞之间的cd99依赖性串扰,增强Th2反应,这表明其具有抗炎作用。相反,pekk相关巨噬细胞引发造血干细胞中icam1驱动的坏死,破坏造血稳态。这些结果表明,早期巨噬细胞相关反应是Ti和PEKK植入物临床结果差异的关键决定因素。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Macrophage-related immune responses to polyetherketoneketone bone implants: Single-cell transcriptome analysis
Polyetherketoneketone (PEKK) has emerged as a potential alternative to titanium (Ti) for bone implants. Nevertheless, its osseointegration performance is inferior to that of Ti, primarily due to the limited understanding of its early immune reactions. To address this limitation, this study utilized single-cell RNA sequencing to investigate the distinct early macrophage responses triggered by Ti-based and PEKK-based implants. This approach enabled the characterization of macrophage-polarization dynamics and intercellular interactions within the bone-marrow microenvironment post-implantation. The findings revealed a material-dependent dichotomy in macrophage phenotype: Ti implants preferentially recruited Cd99+ macrophages, establishing an anti-inflammatory microenvironment that promotes osseointegration. Conversely, PEKK implants recruited Icam1+ macrophages, leading to persistent inflammation and hematopoietic stem cells (HSCs) stress. Additionally, Ti surfaces facilitated CD99-dependent crosstalk between macrophages and T cells, enhancing Th2 responses, which are indicative of an anti-inflammatory effect. In contrast, PEKK-associated macrophages triggered ICAM1-driven necroptosis in HSCs, disrupting hematopoietic homeostasis. These results indicate the early macrophage-related responses as key determinants of the clinical-outcome differences between Ti and PEKK implants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


