肿瘤归巢外泌体能够靶向递送siRNA和异欧前胡素,以克服DLBCL中BTK抑制剂的耐药性
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
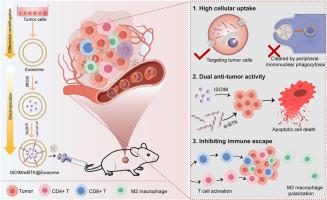
弥漫性大b细胞淋巴瘤(DLBCL)是最常见的淋巴瘤类型,但超过三分之一的患者在一线治疗后复发或发展为难治性疾病。需要新的治疗策略来解决DLBCL持续未满足的临床需求。本研究旨在开发一种基于外泌体的药物传递系统,用于siRNA靶向联合治疗布鲁顿酪氨酸激酶(BTK, B细胞淋巴瘤的既定治疗靶点)和异欧前胡素(ISOIM,一种天然活性呋喃香豆素,具有抗肿瘤作用)治疗DLBCL。分离肿瘤外泌体作为递送载体。通过电穿孔将ISOIM和si-BTK包封在外泌体中制备ISOIM/siBTK@Exosome。在两种DLBCL细胞系和荷瘤小鼠中评估了ISOIM/siBTK@Exosome的细胞摄取、免疫逃逸、靶向递送效率、抗淋巴瘤活性和生物安全性。与单独使用ISOIM@Exosome或siBTK@Exosome相比,ISOIM/siBTK@Exosome具有显著的抗淋巴瘤活性,表明ISOIM和si-BTK具有协同治疗作用。此外,ISOIM/siBTK@Exosome在体外可加速T细胞活化,阻止巨噬细胞M2极化。给药ISOIM/siBTK@Exosome可显著抑制荷瘤小鼠肿瘤生长,延长存活时间。H&;E染色显示ISOIM/siBTK@Exosome具有生物相容性和生物安全性,对主要器官无损伤。制备的ISOIM/siBTK@Exosome可能为DLBCL患者的临床治疗提供新的靶向治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor-homing exosomes enable targeted delivery of siRNA and isoimperatorin for overcoming BTK inhibitor resistance in DLBCL
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma, but over one-third of patients relapse or develop refractory disease after first-line therapy. Novel therapeutic strategies are required to address persistent unmet clinical needs for DLBCL. This study aimed to develop an exosome-based drug delivery system for the targeted combination therapy of siRNA against Bruton's tyrosine kinase (BTK, an established therapeutic target in B cell lymphomas) and isoimperatorin (ISOIM, an active natural furanocoumarin showing anti-tumor effects) in DLBCL. Tumor exosomes were isolated as the delivery carrier. ISOIM/siBTK@Exosome was prepared by encapsulating ISOIM and si-BTK into exosome using electroporation. Cellular uptake, immune escape, targeted delivery efficiency, anti-lymphoma activity and biosafety of ISOIM/siBTK@Exosome were evaluated in two DLBCL cell lines and in tumor-bearing mice. ISOIM/siBTK@Exosome displayed significant anti-lymphoma activity compared to ISOIM@Exosome or siBTK@Exosome alone, demonstrating synergistic therapeutic role of ISOIM and si-BTK. Besides, ISOIM/siBTK@Exosome can accelerate T cells activation and prevent macrophage M2 polarization in vitro. Administration of ISOIM/siBTK@Exosome to tumor-bearing mice significantly inhibited tumor growth and prolonged survival. The ISOIM/siBTK@Exosome was biocompatible and biosafe in vivo without damage on the major organs in H&E staining. The prepared ISOIM/siBTK@Exosome may provide novel targeted therapeutic strategy to be applied in the clinical management of patients with DLBCL.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


