当毒液平息风暴:石鱼的毒液抑制lps诱导的Th1细胞因子的表达和分泌
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
众所周知,毒液可以调节免疫过程。本研究采用逆转录定量聚合酶链式反应(RT-qPCR)、细胞头阵列(CBA)和酶联免疫吸附试验(ELISA)等免疫检测方法,研究了两种石鱼——疣合鱼(synancia verrucosa, SvV)和恐怖鱼(synancia horda, ShV)毒液的免疫调节特性。两种毒液都表现出显著的免疫抑制活性,特别是在脂多糖(LPS)刺激的人外周血单核细胞(pmcs)中,而对离子霉素(P/I)刺激的细胞中12-肉豆蔻酸13-乙酸磷的作用不太明显。这些毒液主要抑制Th1相关细胞因子(TNF、IFN-γ、IL-6和IL-12),以及IL-10 (Th2)和MCP-1,表明对Th1亚群有更强的抑制作用。与ShV相比,SvV表现出更高的活性,可以抑制ShV不作用的细胞因子,并且在浓度低至1.25 μg/mL时具有活性。稳定性研究表明,冷冻和冻干毒液都保留了与新鲜毒液相当的免疫抑制活性,而反相高效液相色谱法(RP-HPLC)完全消除了这种活性。大小排斥层析(SEC)显示,各毒液的早期和晚期部分的免疫抑制活性最强。我们的研究结果强调了疣状葡萄球菌和恐怖葡萄球菌毒液对人类PBMCs的选择性免疫抑制作用,特别是通过对LPS反应中Th1细胞因子的调节。特定毒液组分的稳定性和生物活性强调了它们作为新型免疫治疗剂来源的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
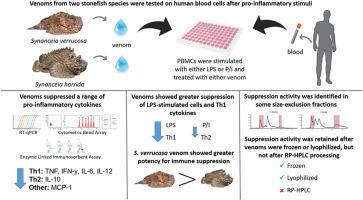
When venom calms the storm: Stonefish venoms suppress LPS-induced Th1 cytokine expression and secretion in human PBMCs
Venoms are known to modulate immunological processes. In this study, we investigated the immunomodulatory properties of venoms from two stonefish species, Synanceia verrucosa (SvV) and Synanceia horrida (ShV), using immunological assays including reverse-transcription quantitative polymerase chain reaction (RT-qPCR), cytometric bead array (CBA), and enzyme-linked immunosorbent assay (ELISA). Both venoms exhibited significant immunosuppressive activity, particularly in lipopolysaccharide (LPS)-stimulated human peripheral blood mononuclear cells (PBMCs), with less pronounced effects on phorbol 12-myristate 13-acetate with ionomycin (P/I)-stimulated cells. The venoms primarily suppressed Th1-associated cytokines (TNF, IFN-γ, IL-6, and IL-12), as well as IL-10 (Th2) and MCP-1, indicating a stronger inhibition of the Th1 subset. SvV demonstrated greater activity compared to ShV, suppressing cytokines on which ShV had no effect, and having activity at concentrations as low as 1.25 μg/mL. Stability studies showed that both frozen and lyophilized venoms retained immunosuppressive activity comparable to fresh venom, while reversed-phase high-performance liquid chromatography (RP-HPLC) abolished this activity entirely. Size-exclusion chromatography (SEC) revealed the immunosuppressive activity was strongest in the early and late fractions of each venom. Our results highlight the selective immunosuppressive effects of S. verrucosa and S. horrida venoms on human PBMCs, particularly via modulation of Th1 cytokines in response to LPS. The stability and bioactivity of specific venom fractions underscore their potential as sources for novel immunotherapeutic agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


