促进铜钼分离:DMTD在浮选过程中的作用
IF 4.6
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
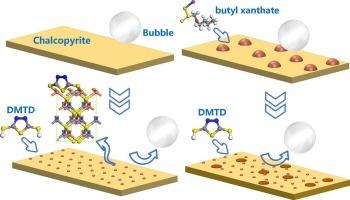
斑岩型铜钼矿中黄铜矿和辉钼矿的分离具有挑战性,因为它们具有相似的可浮性,因此必须使用抑制剂。无机抑制剂通常用于浮选工业,以增加黄铜矿和辉钼矿之间的疏水性差异;然而,这些抑制剂会造成严重的环境风险。近年来,新型有机抑制剂因其丰度高、毒性低而引起了人们的广泛关注。研究了2,5-二巯基-1,3,4-噻二唑(DMTD)作为氧化铜浮选抑制剂对黄铜矿和辉钼矿分离的影响。微浮选实验表明,DMTD具有明显的选择性抑制作用,对黄铜矿的浮选有明显抑制作用,但对辉钼矿的浮选没有不利影响。此外,在黄药存在的情况下,DMTD对黄铜矿浮选仍有抑制作用。通过接触角测量分析了疏水性的变化,证实了微浮选结果,SEM-EDS元素分布图进一步证实了DMTD在黄铜矿表面的选择性吸附。用XPS和AFM分析了DMTD与黄铜矿的微观相互作用机理。通过元素结合能的变化确定了黄铜矿表面的具体相互作用位点,而表面形貌的演变揭示了DMTD在黄药体系中与黄铜矿的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing copper–molybdenum separation: The role of DMTD in flotation processes
The separation of chalcopyrite and molybdenite in porphyry copper–molybdenum ores is challenging owing to their similar floatabilities, making the use of depressants necessary. Inorganic depressants are commonly employed in the flotation industry to increase the difference in hydrophobicity between chalcopyrite and molybdenite; however, these depressants pose significant environmental risks. Recently, novel organic depressants have attracted considerable interest because of their abundance and low toxicity. This study investigated the use of 2,5-dimercapto-1,3,4-thiadiazole (DMTD), an activator recently employed in copper oxide flotation, as a depressant for the separation of chalcopyrite and molybdenite. Micro–flotation experiments demonstrated that DMTD exhibits a notable selective depression effect, significantly suppressing the flotation of chalcopyrite without adversely affecting the flotation of molybdenite. Furthermore, DMTD maintained its depression effect on chalcopyrite flotation even in the presence of xanthate. Contact angle measurements were used to analyze changes in hydrophobicity, corroborating the micro–flotation results, while SEM-EDS elemental distribution maps further confirmed the selective adsorption of DMTD on the chalcopyrite surface. XPS and AFM analyses were conducted to elucidate the microscale interaction mechanism between DMTD and chalcopyrite. The specific interaction sites on the chalcopyrite surface were identified through changes in elemental binding energies, while the evolution of surface morphology revealed how DMTD interacts with chalcopyrite in the xanthate system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Powder Technology
工程技术-工程:化工
CiteScore
9.90
自引率
15.40%
发文量
1047
审稿时长
46 days
期刊介绍:
Powder Technology is an International Journal on the Science and Technology of Wet and Dry Particulate Systems. Powder Technology publishes papers on all aspects of the formation of particles and their characterisation and on the study of systems containing particulate solids. No limitation is imposed on the size of the particles, which may range from nanometre scale, as in pigments or aerosols, to that of mined or quarried materials. The following list of topics is not intended to be comprehensive, but rather to indicate typical subjects which fall within the scope of the journal's interests:
Formation and synthesis of particles by precipitation and other methods.
Modification of particles by agglomeration, coating, comminution and attrition.
Characterisation of the size, shape, surface area, pore structure and strength of particles and agglomerates (including the origins and effects of inter particle forces).
Packing, failure, flow and permeability of assemblies of particles.
Particle-particle interactions and suspension rheology.
Handling and processing operations such as slurry flow, fluidization, pneumatic conveying.
Interactions between particles and their environment, including delivery of particulate products to the body.
Applications of particle technology in production of pharmaceuticals, chemicals, foods, pigments, structural, and functional materials and in environmental and energy related matters.
For materials-oriented contributions we are looking for articles revealing the effect of particle/powder characteristics (size, morphology and composition, in that order) on material performance or functionality and, ideally, comparison to any industrial standard.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


