Sr掺杂TiO2高温相变过程中Sr的动态演化:原位XRD和DFT分析
IF 3.9
2区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
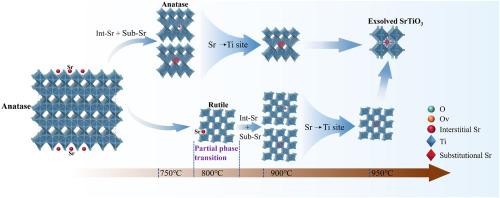
TiO2中锐钛矿-金红石的高温相变已被广泛研究;然而,在此过程中掺杂离子的演变及其对晶格和电子结构的影响仍然知之甚少。本文将高温原位x射线衍射(XRD)数据的半定量Rietveld细化与拉曼光谱、x射线光电子能谱和密度泛函理论计算相结合,系统地阐明了sr掺杂TiO2在750°C到950°C相变过程中的演化过程。750℃时仅观察到SrTiO3(110)反射,800℃时出现金红石;在较高温度下,锐钛矿和金红石与SrTiO3共存。基于纯TiO2的单元胞参数,构建了锐钛矿和金红石中Sr的间隙模型和取代模型。通过差分电荷密度图、Mulliken电荷分析和Ti-O键长计算,首次阐明了相变过程中Sr的演化机制。三阶段演化机制包括:(i)间隙和取代固溶共存;(ii)取代主导的晶格扩展;(iii)固溶饱和后析出形成SrTiO3。本研究为通过Sr掺杂调整TiO2相变温度、晶粒尺寸和缺陷结构提供了理论指导,并对TiO2体系中金属离子的高温行为提供了更深入的了解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Dynamic evolution of Sr during high-temperature phase transition in Sr-doped TiO2: Insights from In Situ XRD and DFT analysis
The high‐temperature anatase-to-rutile phase transition in TiO2 has been extensively investigated; however, the evolution of dopant ions during this process, and their effects on the crystal lattice and electronic structure, remain poorly understood. Herein, we combined semiquantitative Rietveld refinement of high-temperature in situ X-ray diffraction (XRD) data with Raman spectroscopy, X-ray photoelectron spectroscopy, and density functional theory calculations to systematically elucidate the evolution of Sr-doped TiO2 during its phase transition from 750 °C to 950 °C. At 750 °C, only the SrTiO3 (110) reflection was observed, while rutile emerged at 800 °C; at higher temperatures, anatase and rutile coexisted with SrTiO3. Interstitial and substitutional Sr models in anatase and rutile were constructed based on the unit‐cell parameters of pure TiO2. For the first time, the Sr evolution mechanism during the phase transition was clarified through differential charge density maps, Mulliken charge analyses, and Ti–O bond length calculations. The three-stage evolution mechanism includes: (i) interstitial and substitutional solid-solution coexistence, (ii) substitution-dominated lattice expansion, and (iii) solid-solution saturation followed by exsolution to form SrTiO3. This study provides theoretical guidance for tuning TiO2 phase transition temperatures, grain size, and defect structures through Sr doping and provides deeper insights into the high-temperature behavior of metal ions in TiO2 systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Vacuum
工程技术-材料科学:综合
CiteScore
6.80
自引率
17.50%
发文量
0
审稿时长
34 days
期刊介绍:
Vacuum is an international rapid publications journal with a focus on short communication. All papers are peer-reviewed, with the review process for short communication geared towards very fast turnaround times. The journal also published full research papers, thematic issues and selected papers from leading conferences.
A report in Vacuum should represent a major advance in an area that involves a controlled environment at pressures of one atmosphere or below.
The scope of the journal includes:
1. Vacuum; original developments in vacuum pumping and instrumentation, vacuum measurement, vacuum gas dynamics, gas-surface interactions, surface treatment for UHV applications and low outgassing, vacuum melting, sintering, and vacuum metrology. Technology and solutions for large-scale facilities (e.g., particle accelerators and fusion devices). New instrumentation ( e.g., detectors and electron microscopes).
2. Plasma science; advances in PVD, CVD, plasma-assisted CVD, ion sources, deposition processes and analysis.
3. Surface science; surface engineering, surface chemistry, surface analysis, crystal growth, ion-surface interactions and etching, nanometer-scale processing, surface modification.
4. Materials science; novel functional or structural materials. Metals, ceramics, and polymers. Experiments, simulations, and modelling for understanding structure-property relationships. Thin films and coatings. Nanostructures and ion implantation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


