亚变性热预处理调节乳铁蛋白-槲皮素相互作用:结构动力学,结合机制和功能协同
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
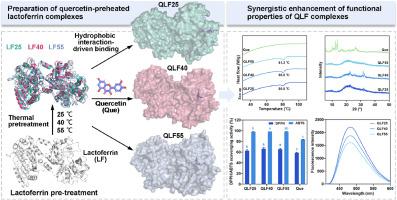
热处理作为蛋白质修饰中最常用的加工方法之一,其影响蛋白质-多酚相互作用的机制尚不清楚。本研究以槲皮素-乳铁蛋白(Que-LF, QLF)体系为模型,研究了亚变性温度预处理对蛋白质结构-功能关系及其与多酚相互作用的影响。结果表明,在25-55℃梯度热处理中,40℃预处理的LF (LF40)通过适度的结构展开形成亚稳聚集体,疏水空腔明显暴露,增强了与Que的结合亲和力(Ka = 7.11 × 105 L/mol)。预热优化了LF结合袋的构像灵活性和空间相容性,使Que能够通过疏水相互作用(ΔH = 23.82 kJ mol−1,ΔS = 188.27 jmol−1·K−1)和多个氢键网络嵌入疏水腔中。QLF40配合物具有最佳的结合自由能(−85.1 kJ/mol)和构象稳定性。此外,Que结合通过屏蔽疏水位点、加强氢键网络和诱导局部结构刚性,协同提高了配合物的热稳定性(变性温度提高2°C)和抗氧化活性(DPPH清除率64.7%和ABTS清除率98.4%)。本研究为设计基于可控蛋白质结构修饰的高效活性成分递送系统提供了理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sub-denaturing thermal pretreatment modulates lactoferrin-quercetin interactions: Structural dynamics, binding mechanisms, and functional synergy
Heat treatment, as one of the most common processing methods in protein modification, has unclear mechanisms affecting protein-polyphenol interactions. This study investigated the effects of sub-denaturing temperature pretreatment on protein structure-function relationships and their interactions with polyphenols using the quercetin-lactoferrin (Que-LF, QLF) system as a model. Results demonstrated that among 25–55 °C gradient heat treatments, LF pretreated at 40 °C (LF40) formed metastable aggregates through moderate structural unfolding, with significantly exposed hydrophobic cavities that enhanced binding affinity to Que (Ka = 7.11 × 105 L/mol). Preheating optimized the conformational flexibility and spatial compatibility of LF binding pockets, enabling Que to embed into hydrophobic cavities via hydrophobic interactions (ΔH = 23.82 kJ mol−1, ΔS = 188.27 J mol−1·K−1) and multiple hydrogen bonding networks. The QLF40 complex exhibited optimal binding free energy (−85.1 kJ/mol) and conformational stability. Furthermore, Que binding synergistically improved thermal stability (2 °C increase in denaturation temperature) and antioxidant activity (64.7 % DPPH and 98.4 % ABTS scavenging rates) of the complex through masking hydrophobic sites, strengthening hydrogen bond networks, and inducing local structural rigidity. This study provides theoretical foundation for designing efficient active ingredient delivery systems based on controlled protein structural modification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


