共萃取的酚类化合物对菜籽蛋白的凝胶性质几乎没有影响
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
菜籽蛋白是一种很有前途的动物蛋白替代品,可以形成凝胶,在食物中提供结构。然而,在植物蛋白的情况下,共提取的杂质,如酚类化合物,可能会干扰蛋白质网络。油菜籽中的酚类化合物主要以籽粒中的辛酸和阿魏酸的形式存在,而在籽壳中以原花青素的形式存在。这些可以与蛋白质共同提取,并可能影响蛋白质凝胶性质。因此,本研究阐明了酚类物质对蛋白质凝胶性质的潜在影响。将提取的油菜籽分离蛋白与辛酸、阿魏酸或原花青素按自然比例在pH 7或9下混合,定量测定加热后得到的凝胶中酚类化合物对其流变性能的影响。将混合蛋白质和酚类化合物制成的凝胶与天然蛋白质提取物制成的凝胶进行比较,其中天然存在辛酸,阿魏酸和原花青素。剪切流变仪显示只有微小的差异,凝胶的流变行为与蛋白质-酚醛混合物。此外,在凝胶化之前,无论环境pH值如何,都只观察到蛋白质分散性或交联的微小差异,这表明蛋白质和酚类物质之间的相互作用很弱。与通常的做法相反,不需要从蛋白质提取物中去除酚类化合物,这使得蛋白质提取步骤更简单,从而使蛋白质提取过程在成本和能源方面更有效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
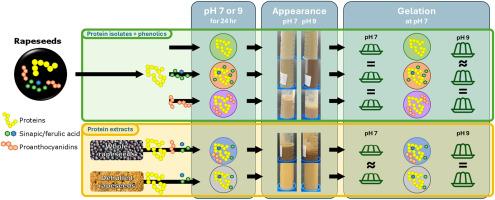
The gelation properties of rapeseed proteins are barely affected by co-extracted phenolic compounds
Rapeseed proteins are a promising alternative to animal-based proteins that can form gels to provide structure in foods. However, in the case of plant proteins, co-extracted impurities, such as phenolic compounds, may interfere with the protein network. Phenolic compounds in rapeseeds are found mainly in the form of sinapic and ferulic acid in the kernels and as proanthocyanidins in the hulls. These can be co-extracted with the proteins and may affect the protein gel properties. Therefore, the current study elucidates this potential effect of phenolics on the protein gel properties. An extracted rapeseed protein isolate was mixed with sinapic and ferulic acid or with proanthocyanidins in a natural ratio at pH 7 or 9, and the effects of the phenolic compounds on the rheological properties of the gel obtained after heating were quantified. The gels made by mixing proteins and phenolic compounds were compared with gels made up of natural protein extracts where sinapic acid, ferulic acid, and proanthocyanidins were naturally present. Shear rheometry revealed only minor differences in the rheological behaviour of the gels with protein-phenolic blends. Furthermore, only minor differences in protein dispersibility or cross-linking were observed before gelation, regardless of the environmental pH, indicating weak interactions between proteins and phenolics. In contrast to common practice, phenolic compounds do not need to be removed from the protein extracts, which enables protein extraction in more simple steps, thereby making the protein extraction process more efficient, in terms of costs and energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


