肿瘤组织源性外泌体作为靶向抗癌治疗的生物纳米载体的潜力
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-09-08
DOI:10.1016/j.jddst.2025.107503
引用次数: 0
摘要
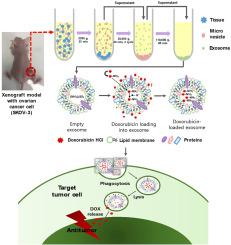
肿瘤组织源性外泌体(tdEXOs)已成为个性化癌症治疗的有前途的纳米载体,提供了反映肿瘤微环境的天然膜成分。本研究直接从患者来源的卵巢肿瘤组织中分离tdEXOs,并采用硫酸铵梯度法负载多柔比星(DOX)。得到的DOX-tdEXOs包封效率高,保持了囊泡形态,物理化学性质稳定。与游离DOX相比,DOX- tdexos在SKOV-3卵巢癌细胞中表现出增强的细胞摄取和细胞毒性。tdEXOs也被同源癌细胞选择性摄取,提示其存在一种由膜蛋白保存介导的同型靶向机制。体内生物分布成像进一步显示tdEXOs在全身给药后在肿瘤组织中优先积累。这些结果支持了tdexo作为自体、来源特异性递送载体的可行性,并强调了它们在减少脱靶效应的同时提高治疗效果的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Potential of tumor tissue-derived exosomes as promising bionanocarriers for targeted anticancer therapy
Tumor tissue-derived exosomes (tdEXOs) have emerged as promising nanocarriers for personalized cancer therapy, offering native membrane compositions that reflect the tumor microenvironment. In this study, tdEXOs were isolated directly from patient-derived ovarian tumor tissues and loaded with doxorubicin (DOX) using an ammonium sulfate gradient method. The resulting DOX-tdEXOs exhibited high encapsulation efficiency, preserved vesicular morphology, and stable physicochemical properties. Compared to free DOX, DOX-tdEXOs demonstrated enhanced cellular uptake and cytotoxicity in SKOV-3 ovarian cancer cells. The tdEXOs also showed selective uptake by homologous cancer cells, suggesting a homotypic targeting mechanism mediated by membrane protein preservation. In vivo biodistribution imaging further revealed preferential accumulation of tdEXOs in tumor tissues following systemic administration. These results support the feasibility of tdEXOs as autologous, origin-specific delivery vehicles and underscore their potential to improve therapeutic efficacy while minimizing off-target effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


