亚急性暴露2,2 ',4,4 ' -四溴联苯醚通过抑制线粒体自噬和增加NLRP3炎性体诱导小鼠肝损伤
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
2 ',4,4 ' -四溴二苯醚(BDE-47)是在环境中发现的最常见的溴化阻燃剂之一,已被证明与各种不利的人类健康影响有关。它已被证明会产生肝毒性,但对接触BDE-47后的潜在机制知之甚少。本研究通过灌胃BDE-47 (12.5-100mg /kg/天)4周,研究了BDE-47对小鼠的肝毒性。结果显示,BDE-47暴露小鼠血清中ALT和AST含量升高,肝脏组织病理改变。此外,我们观察到随着BDE-47剂量的增加,线粒体膜电位(MMP)降低,ROS升高。BDE-47还上调NLRP3蛋白水平,上调caspase-1、IL-1β、IL-18和TNF-α炎症因子的蛋白表达。此外,BDE-47暴露小鼠肝脏中Parkin和PINK1蛋白表达降低,P62蛋白表达升高,线粒体自噬被阻断。然而,自噬激活剂雷帕霉素(rapamycin, RAPA)激活线粒体自噬,通过增加PINK1和Parkin的蛋白表达,降低NLRP3和炎症因子,减轻了BDE-47诱导的小鼠肝损伤。上述结果提示,bde -47暴露小鼠NLRP3炎性小体激活介导的线粒体和肝脏损伤是由于线粒体自噬下调所致,激活线粒体自噬可以通过调节NLRP3炎性小体减轻bde -47诱导的炎症损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
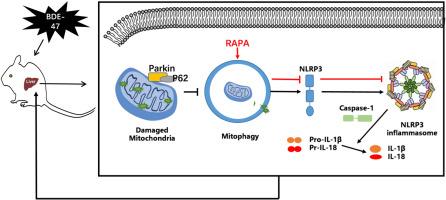
Subacute exposure of 2, 2′, 4, 4′-tetrabromodiphenyl ether induced liver injury by inhibiting mitochondrial autophagy and increasing NLRP3 inflammasome in mice
2′,4,4′-tetrabrominated diphenyl ethers (BDE-47) is one of the most common brominated flame retardants found in environment and has been demonstrated to be associated with a variety of adverse human health effects. It has been proven to generate liver toxicity, but little is known about the potential mechanism following BDE-47 exposure. Here, hepatotoxicity of BDE-47 in mice was investigated by gavage exposure to BDE-47 (12.5–100mg/kg/day) for 4 weeks. The results showed the increasing of ALT and AST in serum and histopathological change of liver in BDE-47 exposure mice. In addition, we observed that the mitochondrial membrane potential (MMP) decreased and ROS increased with the increase of BDE-47 dose. BDE-47 also up-regulated NLRP3 protein levels, and proteins expression of caspase-1, IL-1β, IL-18 and TNF-α inflammatory factors. Furthermore, mitophagy was blocked with Parkin and PINK1 protein expression decrease and P62 increase in liver of BDE-47 exposure mice. However, activation of mitophagy with autophagy activator rapamycin (RAPA) alleviated liver injury induced by BDE-47 in mice via increasing the protein expression of PINK1 and Parkin, and decreasing NLRP3 and inflammatory factors. These results suggest NLRP3 inflammasome activation-mediated mitochondrial and liver damage in BDE-47-exposed mice is due to mitophagy downregulation, and activating mitophagy can alleviate BDE-47-induced inflammatory damage by regulating NLRP3 inflammasome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


