生物工程改进三维血管化共培养模型用于研究神经元-小胶质细胞相互作用
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
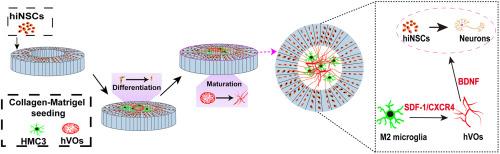
神经元、小胶质细胞和内皮细胞(ECs)——神经血管单元(NVU)的主要组成部分——之间的相互作用对于维持中枢神经系统(CNS)稳态至关重要,并与许多神经系统疾病有关。然而,由于缺乏与生理相关的体外模型,对其串扰的机制见解仍然有限。在这项研究中,我们提出了一种改进的三维血管化三培养模型,该模型将人类诱导的神经干细胞(hiNSCs)、人类血管类器官(hVOs)和小胶质细胞整合在几何工程丝素蛋白支架中。该平台有效地概括了原生中枢神经系统微环境的关键特征,包括空间神经血管模式和细胞类型特异性相互作用。在该模型中,hVOs显著促进hiNSCs的神经元分化,导致轴突网络延长,神经血管排列改善。发现小胶质细胞的作用是表型依赖的:静息(M0)和促炎(M1)小胶质细胞都抑制hiNSCs的分化和血管发育,其中M1细胞的抑制作用最强。相反,抗炎(M2)小胶质细胞表现出最小的抑制作用,甚至适度支持神经血管成熟。机制研究表明M2小胶质细胞通过基质细胞衍生因子1 (SDF-1)/C-X-C趋化因子受体4 (CXCR4)信号轴与hVOs协同促进神经元分化。据我们所知,这是在人类三培养系统中首次证明SDF-1/ cxcr4介导的免疫-神经血管相互作用。此后,这种三维血管化共培养模型为研究神经免疫和神经血管相互作用提供了一个生理相关的体外平台。它在神经发育和神经变性的机制研究、药物评估和再生疗法的发展方面具有广泛的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Bioengineering an improved three-dimensional vascularized co-culture model for studying Neuron–Microglia interactions
Interactions among neurons, microglia, and endothelial cells (ECs) —the principal components of the neurovascular unit (NVU)—are vital for maintaining central nervous system (CNS) homeostasis and are implicated in numerous neurological disorders. However, mechanistic insights into their crosstalk remain limited due to the lack of physiologically relevant in vitro models. In this study, we present an improved 3D vascularized tri-culture model that integrates human-induced neural stem cells (hiNSCs), human vascular organoids (hVOs), and microglia within a geometrically engineered silk fibroin scaffold. This platform effectively recapitulates critical features of the native CNS microenvironment, including spatial neurovascular patterning and cell-type-specific interactions. Within this model, hVOs significantly promoted neuronal differentiation of hiNSCs, resulting in extended axonal networks and improved neurovascular alignment. Microglial effects were found to be phenotype-dependent: both resting (M0) and pro-inflammatory (M1) microglia inhibited hiNSCs differentiation and vascular development, with M1 cells exerting the strongest suppressive influence. In contrast, anti-inflammatory (M2) microglia displayed the least inhibitory effect and even modestly supported neurovascular maturation. Mechanistic studies revealed that M2 microglia cooperate with hVOs via the stromal cell-derived factor 1 (SDF-1)/C-X-C chemokine receptor type 4 (CXCR4) signaling axis to promote neuronal differentiation. To our knowledge, this represents the first demonstration of SDF-1/CXCR4-mediated immune-neurovascular interaction within a human tri-culture system. Thereafter, this 3D vascularized co-culture model provides a physiologically relevant in vitro platform to investigate neuroimmune and neurovascular interactions. It holds broad potential for mechanistic studies in neurodevelopment and neurodegeneration, drug evaluation, and the development of regenerative therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


