安慰剂效应对MASH患者磁共振终点变化的影响:系统评价和荟萃分析
IF 3.2
Q2 GASTROENTEROLOGY & HEPATOLOGY
Journal of Clinical and Experimental Hepatology
Pub Date : 2025-08-23
DOI:10.1016/j.jceh.2025.103173
引用次数: 0
摘要
背景和目的基于磁共振(MR)的技术越来越多地被用于代谢功能障碍相关脂肪性肝炎(MASH)的早期试验中,作为一种非侵入性的肝脏组织学方法来评估药物疗效。了解基于核磁共振的终点的安慰剂效应对于解释结果和设计未来的试验至关重要。我们进行了一项系统回顾和荟萃分析,以量化安慰剂反应并确定其基于mr的终点的修饰因子。方法:我们系统检索PubMed和Cochrane Library,检索截止到2024年12月13日的≥2期随机对照试验(rct),比较药物与安慰剂对成人MASH患者的影响,提供基于mr的评估。用Clopper-Pearson区间的广义线性混合模型估计安慰剂的综合反应率。结果我们纳入了21项随机对照试验,包括800名安慰剂治疗的患者。19.21% (95% CI: 16.23 ~ 22.59; I2 = 7.2%)安慰剂患者肝脂肪相对减少(磁共振成像[MRI]质子密度脂肪分数[MRI- pdff]相对减少≥30%)具有临床意义。肝脂肪正常化(MRI-PDFF绝对降低≥5%)和MRI-PDFF超缓解(MRI-PDFF相对降低≥50%)的安慰剂综合缓解率分别为17.19% (95% CI: 11.70至24.56;I2 = 27.9%)和6.14% (95% CI: 2.63至13.69;I2 = 12.9%)。肝脏硬度降低(磁共振弹性成像相对降低≥15%)和纤维炎症(多参数MRI校正T1反应)分别为26.09% (95% CI: 18.14至35.98;I2 = 15.1%)和13.43% (95% CI: 7.14至23.85;I2 = 0%)。结论:安慰剂治疗的MASH患者在基于mr的终点均有显著改善,异质性低。这些数据支持基于核磁共振的终点在评估MASH治疗疗效方面的潜在可重复性,并应在未来的试验设计中予以考虑。本文章由计算机程序翻译,如有差异,请以英文原文为准。
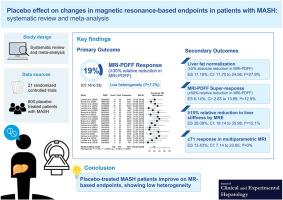
Placebo Effect on Changes in Magnetic Resonance-based Endpoints in Patients With MASH: Systematic Review and Meta-analysis
Background and aims
Magnetic resonance (MR)-based techniques are increasingly being used in early-phase trials of metabolic dysfunction-associated steatohepatitis (MASH) as a noninvasive alternative to liver histology for assessing drug efficacy. Understanding the placebo effect in MR-based endpoints is essential for interpreting results and designing future trials. We conducted a systematic review and meta-analysis to quantify the placebo response and identify its modifiers in MR-based endpoints.
Methods
We systematically searched PubMed and the Cochrane Library through December 13, 2024 for phase ≥2 randomized controlled trials (RCTs) comparing pharmacologic agents with placebo in adults with MASH that provided MR-based assessments. Pooled placebo response rates were estimated using generalized linear mixed models with Clopper–Pearson intervals.
Results
We included 21 RCTs comprising 800 placebo-treated patients. A clinically meaningful relative reduction in liver fat (≥30% relative reduction in magnetic resonance imaging [MRI] proton density fat fraction [MRI-PDFF]) was observed in 19.21% (95% CI: 16.23 to 22.59; I2 = 7.2%) of placebo patients. The pooled placebo response rates for liver fat normalization (≥5% absolute reduction in MRI-PDFF) and MRI-PDFF super-response (≥50% relative reduction in MRI-PDFF) were 17.19% (95% CI: 11.70 to 24.56; I2 = 27.9%) and 6.14% (95% CI: 2.63 to 13.69; I2 = 12.9%), respectively. Reductions in liver stiffness (≥15% relative reduction by magnetic resonance elastography) and fibro-inflammation (corrected T1 response by multiparametric MRI) occurred in 26.09% (95% CI: 18.14 to 35.98; I2 = 15.1%) and 13.43% (95% CI: 7.14 to 23.85; I2 = 0%), respectively.
Conclusions
Placebo-treated patients with MASH show significant improvements across MR-based endpoints with low heterogeneity. These data support the potential reproducibility of MR-based endpoints in assessing treatment efficacy in MASH and should be considered in future trial design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Clinical and Experimental Hepatology
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
4.90
自引率
16.70%
发文量
537
审稿时长
64 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


