一种基于雷鲍迪苷A的新型纳米微球:一种增强杨梅素肾保护作用的口服纳米平台
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
顺铂诱导的急性肾损伤(AKI)是一个重大的临床挑战,主要以炎症反应和氧化应激为特征。本研究旨在开发一种杨梅素(Myr)负载雷鲍迪苷a (RA)纳米胶束递送系统(RA-Myr),并研究其体外和体内肾保护作用。采用薄膜水化法制备了RA-Myr纳米胶束。RA-Myr的表征包括评价粒径、包封效率和稳定性。使用FRAP法评估RA- myr的抗氧化能力,并使用香豆素6负载RA纳米胶束评估细胞摄取。在HK-2细胞和雄性昆明小鼠中研究RA-Myr对顺铂诱导AKI的保护作用及其可能机制。RA-Myr显著抑制顺铂诱导的HK-2细胞增殖抑制,减少ROS积累,恢复线粒体膜电位。在体内,RA-Myr减轻了顺铂诱导的AKI,表现为血尿素氮(BUN)和血清肌酐(SCr)水平降低,并减轻了肾组织病理损伤。在机制上,RA-Myr保护顺铂诱导的DNA损伤并抑制cGAS-STING途径。RA-Myr纳米粒递送系统有望通过增强Myr的肾保护作用来缓解顺铂诱导的AKI。本文章由计算机程序翻译,如有差异,请以英文原文为准。
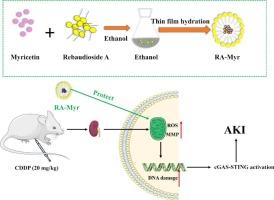
A novel nanomicelle based on Rebaudioside A: An oral nanoplatform with enhanced nephroprotective effect of myricetin
Cisplatin-induced acute kidney injury (AKI) is a significant clinical challenge, primarily characterized by inflammatory responses and oxidative stress. This study aimed to develop a myricetin (Myr) loaded Rebaudioside A (RA) nanomicelle delivery system (RA-Myr) and investigate its nephroprotective effects both in vitro and in vivo. RA-Myr nanomicelles were prepared using a thin film hydration method. The characterization of RA-Myr included evaluating particle size, encapsulation efficiency, and stability. The antioxidant capacity of RA-Myr was assessed using the FRAP assay, and cellular uptake was evaluated using coumarin 6-loaded RA nanomicelles. The protective effects and potential mechanisms of RA-Myr on cisplatin-induced AKI were studied in HK-2 cells and male Kunming mice. RA-Myr significantly inhibited cisplatin-induced suppression of HK-2 cell proliferation, reduced ROS accumulation, and restored mitochondrial membrane potential. In vivo, RA-Myr alleviated cisplatin-induced AKI, evidenced by decreased blood urea nitrogen (BUN) and serum creatinine (SCr) levels, and mitigated kidney tissue pathological damage. Mechanistically, RA-Myr protected against cisplatin-induced DNA damage and inhibited the cGAS-STING pathway. The RA-Myr nanomicelle delivery system shows promise as a potential strategy for alleviating cisplatin-induced AKI by enhancing the nephroprotective effects of Myr.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


