采用模拟回转窑-电炉(RKEF)技术,采用甲烷-氩气混合物还原腐岩镍矿
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
腐岩镍矿通常采用一种称为回转窑-电炉(RKEF)的火法冶金工艺进行加工,这种工艺约占红土镍矿镍产量的75%。在RKEF技术中,煤扮演着还原剂、燃料和电源的多重角色。甲烷是镍工业减少二氧化碳排放的还原剂和燃料的可行选择,特别是在氢气生产规模扩大之前作为权宜之计。然而,在镍生产中使用甲烷作为还原剂的研究有限。本研究旨在通过实验室规模的RKEF利用甲烷气体从腐岩镍矿中生产镍铁。最初,FactSage 8.2用于研究该过程的热力学。分两个阶段进行了一系列的室内实验,模拟了RKEF过程。在每次煅烧物在1550°C下熔化2小时之前,它涉及改变还原温度(500-900°C),还原时间(15-120分钟)和气体成分(25和75 vol% CH4/Ar)。所获得的金属平均成分为71.3% Fe和14.3% Ni。结果表明,甲烷可用于生产镍铁,其组成与工业镍铁相似。本文章由计算机程序翻译,如有差异,请以英文原文为准。
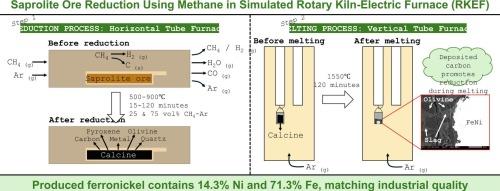
Reduction of saprolite nickel ore using methane-argon gas mixture with laboratory-scale simulated rotary kiln-electric furnace (RKEF) technology
Saprolite nickel ore is typically processed using a pyrometallurgical route called Rotary Kiln-Electric Furnace (RKEF), which contributes to about 75 % of nickel production from laterite nickel ores. In RKEF technology, coal plays multiple roles as a reductant, fuel, and electricity source. Methane is a viable option as reductant and fuel for reducing CO2 emissions in the nickel industry, particularly as a stopgap measure until hydrogen production can be scaled up. However, there has been limited research on using methane as a reductant in nickel production. This study aims to produce ferronickel from saprolite nickel ore through a laboratory-scale RKEF using methane gas. Initially, FactSage 8.2 was used to investigate the thermodynamics of the process. A series of laboratory experiments were carried out in two stages to simulate the RKEF process. It involved varying the reduction temperature (500–900 °C), reduction time (15–120 min), and gas composition (25 and 75 vol% CH4/Ar) before each calcine was melted at 1550 °C for 2 h. The average composition of metals obtained was 71.3 % Fe and 14.3 % Ni. The results showed that methane can be used to produce ferronickel with a similar composition as commercial ferronickel.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


