新型α-葡萄糖苷酶抑制剂二芳基戊烷衍生物的合成及其抑制机制和降糖作用
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
以高血糖为特征的糖尿病(DM)是由于胰岛素缺乏、胰岛素功能障碍或两者兼有。α-葡萄糖苷酶是糖尿病公认的治疗靶点,其抑制剂可有效缓解餐后高血糖,尤其是2型糖尿病。本研究设计并合成了一系列二芳戊烷衍生物作为α-葡萄糖苷酶的潜在抑制剂。体外酶分析表明,对取代衍生物对α-葡萄糖苷酶具有良好的抑制活性,在100 μM范围内抑制率为23.3% ~ 75.7%。化合物5c的IC50值为18.1 μM,是阿卡波糖(IC50 = 312.0 μM)的17倍。5c的抑制活性受到大分子拥挤效应的高度影响,尤其是聚乙二醇(PEG)诱导的分子量依赖性药效降低。酶动力学、分子对接和光谱分析表明,5c通过氢键、静电相互作用和疏水作用与α-葡萄糖苷酶结合,导致α-葡萄糖苷酶二级结构发生明显的构象改变。这些变化包括α-螺旋和β-片之间的转变,以及表面疏水性的降低,从而增强了酶的结构刚性,稳定了酶的二级结构,最终导致α-葡萄糖苷酶的可逆抑制。体内药理学评估表明,5c剂量依赖性地减轻了淀粉/蔗糖刺激小鼠的餐后高血糖,在40 mg/kg的剂量下,血糖AUC降低了29.4%和21.7%。计算ADME分析证实5c符合Lipinski规则,表明良好的口服生物利用度。这些结果共同确定5c是一种治疗2型糖尿病的有前途的α-葡萄糖苷酶口服抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。

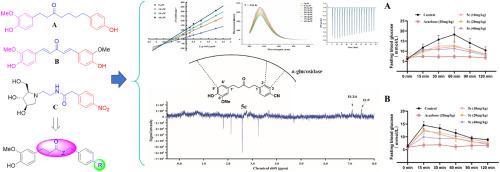
Synthesis of diarylpentane derivatives as novel α-glucosidase inhibitors: Their inhibitory mechanism and hypoglycemic effects
Diabetes mellitus (DM) characterized by hyperglycemia is due to insulin deficiency, insulin dysfunction, or both. α-Glucosidase is a well-established therapeutic target for DM, and its inhibitors can effectively mitigate postprandial hyperglycemia, particularly in T2DM. In this study, a series of diarylpentane derivatives were designed and synthesized as potential α-glucosidase inhibitors. In vitro enzymatic assays showed that the para-substituted derivatives exhibited superior inhibitory activity against α-glucosidase, with inhibition rates ranging from 23.3 % to 75.7 % at 100 μM. Notably, compound 5c demonstrated the most potent inhibition with an IC50 value of 18.1 μM, approximately 17-fold more potent than acarbose (IC50 = 312.0 μM). The inhibitory activity of 5c is highly affected by macromolecular crowding effects, especially polyethylene glycol (PEG) inducing a molecular weight-dependent efficacy reduction. Mechanism study through enzyme kinetics, molecular docking and spectroscopic analyses indicated that 5c binds with α-glucosidase via hydrogen bonds, electrostatic interactions and hydrophobic effects, which induces significant conformational alterations in the secondary structure of α-glucosidase. These changes includes transition between α-helix and β-sheet, and decrease of the surface hydrophobicity to enhance structural rigidity and stabilize secondary structure of the enzyme, ultimately leading to reversible inhibition of α-glucosidase. In vivo pharmacological evaluation demonstrated that 5c dose-dependently attenuated postprandial hyperglycemia in starch/sucrose-challenged mice, achieving 29.4 % and 21.7 % reductions in glycemic AUC at a dose of 40 mg/kg. Computational ADME profiling confirmed that 5c complied with Lipinski's rules, suggesting favorable oral bioavailability. These results collectively identify 5c as a promising oral inhibitor of α-glucosidase for the treatment of T2DM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


