壳寡糖纳米颗粒双STING激活和CD47/SIRPα阻断增强抗肿瘤免疫
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
干扰素基因刺激因子(STING)途径是癌症免疫治疗的一个有希望的靶点,但传统的小分子激动剂存在药代动力学差和全身毒性的问题。为了解决这个问题,我们设计了壳寡糖(COS)-树突状聚碳酸酯(DPC)纳米颗粒(CD NPs)作为具有增强稳定性和肿瘤保留性的聚合STING激动剂。与游离COS相比,CD NPs诱导巨噬细胞和肿瘤细胞中Ifnb1和Cxcl10表达升高~5 - 7倍,强烈激活TBK1/IRF3磷酸化,延长STING信号通路。在体内,CD NPs选择性地在肿瘤中积累,增加M1巨噬细胞和树突状细胞成熟,减少髓源性抑制细胞,促进CD8+ t细胞浸润,从而将肿瘤微环境重塑为促炎状态。为了进一步对抗免疫逃避,我们将CD47/SIRPα抑制剂RRX-001包封到活性氧(ROS)应答载体中,得到RRX@RCD。这种双模平台在放大STING信号的同时阻断了“不要吃我”信号,显著增强了肿瘤细胞的吞噬和干扰素的产生。在CT26荷瘤小鼠中,RRX@RCD实现了优越的肿瘤消退,中位生存期翻了一番。总体而言,RRX@RCD同步先天和适应性免疫激活,为持久的抗肿瘤免疫提供了安全有效的纳米药物策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
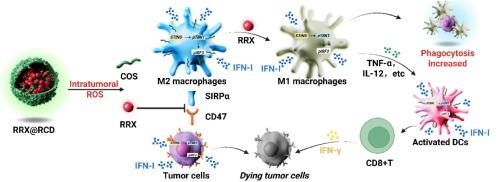
Dual STING activation and CD47/SIRPα blockade via chitooligosaccharide-based nanoparticles to amplify antitumor immunity
The stimulator of interferon genes (STING) pathway represents a promising target for cancer immunotherapy, but traditional small-molecule agonists suffer from poor pharmacokinetics and systemic toxicity. To address this, we engineered chitooligosaccharide (COS)-dendritic polycarbonate (DPC) nanoparticles (CD NPs) as polymeric STING agonists with enhanced stability and tumor retention. Compared with free COS, CD NPs induced ∼5–7-fold higher Ifnb1 and Cxcl10 expression, robustly activated TBK1/IRF3 phosphorylation, and strengthened STING signaling in macrophages and tumor cells. In vivo, CD NPs selectively accumulated in tumors, increased M1 macrophages and dendritic cell maturation, reduced myeloid-derived suppressor cells, and promoted CD8+ T-cell infiltration, thereby reshaping the tumor microenvironment into a pro-inflammatory state. To further counter immune evasion, we encapsulated the CD47/SIRPα inhibitor RRX-001 into reactive oxygen species (ROS)-responsive carriers, yielding RRX@RCD. This dual-modality platform blocked the “don't eat me” signal while amplifying STING signaling, markedly enhancing tumor cell phagocytosis and interferon production. In CT26 tumor-bearing mice, RRX@RCD achieved superior tumor regression, doubled median survival. Overall, RRX@RCD synchronizes innate and adaptive immune activation, offering a safe and potent nanomedicine strategy for durable antitumor immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


