原位形成丝素蛋白水凝胶敷料通过免疫调节和细胞外基质再生加速急性伤口愈合
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
皮肤损伤,一个普遍的临床挑战,往往面临延迟愈合由于不理想的伤口管理策略。传统的水凝胶敷料受到机械性能不足、生物活性差和治疗效果不足的限制。本研究将具有可调结构和生物活性的天然生物聚合物丝素(SF)与聚乙烯醇丁醛(PVB)结合,开发了一种创新的原位成形水凝胶敷料,为创面愈合提供了理想的活性物质输送系统,在皮肤创面治疗中具有广阔的应用前景。乙醇蒸发后,SF/PVB复合材料迅速形成具有增强机械强度、最佳透气性和防水性的水凝胶膜。基于sf的敷料(LD-SF)显示出多功能伤口愈合能力,包括快速止血、抗氧化活性和抗炎调节。值得注意的是,观察到分子量(MW)依赖的生物活性:低分子量SF(45 kDa)显著促进成纤维细胞增殖和迁移,而高分子量SF(72 kDa)通过极化巨噬细胞向促分化表型表现出优异的免疫调节作用。在小鼠全层伤口模型中,LD-SF加速了再上皮化,增强了血管生成,并刺激了胶原重塑。从机制上讲,LD-SF通过β-sheet驱动的结构稳定性和氨基酸介导的代谢支持促进细胞外基质再生。这种双作用系统协同协调免疫调节——通过巨噬细胞表型调节和细胞因子平衡——和强大的细胞外基质再生,为减少疤痕和加速功能恢复的急性伤口修复提供了一种变革性的方法。该研究通过整合快速原位膜形成、分子量可调生物活性和疤痕最小化结果,为急性伤口护理提供了临床可翻译的解决方案,从而解决了当前伤口管理技术的关键空白。本文章由计算机程序翻译,如有差异,请以英文原文为准。
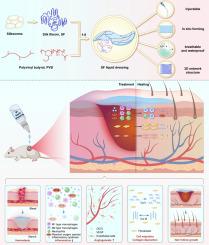
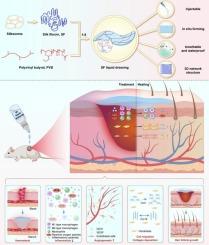
In situ forming silk fibroin hydrogel dressing accelerates acute wound healing via immunomodulation and extracellular matrix regeneration
Cutaneous injuries, a prevalent clinical challenge, often face delayed healing due to suboptimal wound management strategies. Conventional hydrogel dressings are limited by inadequate mechanical properties, poor bioactivity, and insufficient therapeutic efficacy. Here, we developed an innovative in situ forming hydrogel dressing by integrating silk fibroin (SF)—a natural biopolymer with tunable structural and bioactive properties—with polyvinyl butyral (PVB), which provides an ideal system for the delivery of active substances for wound healing and has broad application prospects in skin wound management. Upon ethanol evaporation, the SF/PVB composite rapidly formed a hydrogel film with enhanced mechanical strength, optimal breathability, and waterproofness. The SF-based dressing (LD-SF) demonstrated multifunctional wound-healing capabilities, including rapid hemostasis, antioxidative activity, and anti-inflammatory modulation. Notably, molecular-weight (MW)-dependent bioactivity was observed: low-MW SF (45 kDa) significantly promoted fibroblast proliferation and migration, while high-MW SF (72 kDa) exhibited superior immunomodulatory effects by polarizing macrophages toward pro-resolving phenotypes. In a murine full-thickness wound model, LD-SF accelerated re-epithelialization, enhanced angiogenesis, and stimulated collagen remodeling. Mechanistically, LD-SF facilitated extracellular matrix regeneration via β-sheet-driven structural stability and amino acid-mediated metabolic support. This dual-action system synergistically orchestrates immunomodulation—through macrophage phenotype regulation and cytokine balance—and robust extracellular matrix regeneration, offering a transformative approach to acute wound repair with minimized scarring and accelerated functional recovery. The study provides a clinically translatable solution for acute wound care by integrating rapid in situ film formation, molecular-weight-tunable bioactivity, and scar-minimizing outcomes, thereby addressing critical gaps in current wound management technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


