紫外光酪氨酸酶级联笼反义寡核苷酸精确治疗色素沉着
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
色素沉着是一种以黑色素异常积累为特征的皮肤疾病,其中黑色素皮质素-1受体(melanocortin-1 receptor, MC1R)被认为是黑色素生成的关键靶点。反义寡核苷酸(ASO)通过靶向mRNA抑制蛋白质合成,是一种很有前景的基因治疗色素沉着症的方法。然而,非特异性激活、脱靶毒性和有限的细胞渗透性等挑战阻碍了它们更广泛的临床应用。受中国谚语“化敌为友”的启发,我们介绍了一种双门、紫外(UV)-酪氨酸酶(TYR)级联响应的改性ASO (m-ASO),可以实现时空控制的MC1R沉默。工程m-ASO通过胸苷修饰笼化,使其失活,直到依次暴露于外源紫外线和细胞内TYR。紫外线照射,传统的色素沉着触发,被重新利用,以启动老化m-ASO到中间形式,pH-ASO。然后,这种中间体被色素组织内黑色素细胞中过表达的TYR酶激活,最终恢复ASO的天然构象和治疗功能。这种“从敌人到盟友”的策略允许针对特定疾病,精确激活ASO疗法。值得注意的是,为了增强透皮给药,m-ASO纳米复合物被整合到一个生物相容性的、可溶解的微针系统中,用于无痛和局部表皮给药。由此产生的平台显示精确的MC1R沉默和有效的黑色素抑制,同时保留正常的黑色素细胞。总之,这种级联笼式、细胞选择性和微创策略为皮肤疾病和酶过表达疾病的可编程核酸治疗提供了一个新的框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。

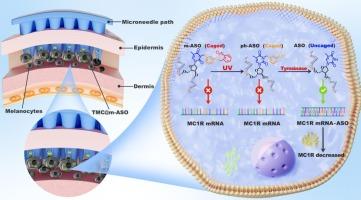
Ultraviolet-tyrosinase cascade caged antisense oligonucleotide for precise treatment of hyperpigmentation
Hyperpigmentation is a skin disorder characterized by the abnormal accumulation of melanin, in which the melanocortin-1 receptor (MC1R) was recognized as a key target for melanin production. Antisense oligonucleotide (ASO), by targeting mRNA to inhibit protein synthesis, holds a promising gene therapy for hyperpigmentation. However, challenges such as nonspecific activation, off-target toxicity, and limited cellular permeability hinder their broader clinical application. Inspired by the Chinese proverb “Turn an enemy into a friend”, we introduce a dual-gated, ultraviolet (UV)-tyrosinase (TYR) cascade-responsive modified ASO (m-ASO) that enables spatiotemporally controlled silencing of MC1R. The engineered m-ASO is caged via thymidine modification, rendering it inactive until sequentially exposed to exogenous UV and intracellular TYR. UV irradiation, conventionally hyperpigmentation trigger, is repurposed to initiate decaging of m-ASO into an intermediate form, ph-ASO. This intermediate is then enzymatically activated by TYR overexpressed in melanocytes within pigmented tissues, ultimately restoring the native conformation and therapeutic function of the ASO. This “enemy-to-ally” strategy allows for disease-specific, precision activation of ASO therapeutics. Notably, to enhance transdermal delivery, the m-ASO nanocomplex is integrated into a biocompatible, dissolvable microneedle system for painless and localized epidermal administration. The resulting platform demonstrates precise MC1R silencing and effective melanin suppression, while sparing normal melanocytes. Together, this cascade-caged, cell-selective, and minimally invasive strategy presents a novel framework for programmable nucleic acid therapeutics in dermatological and enzyme-overexpressing diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


