声波触发纳米放大器介导细菌性肺炎的铁中毒免疫调节
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在大流行后时代,耐多药(MDR)细菌性肺炎因其经常逃避常规抗生素治疗而成为一项重大的全球卫生挑战。氧化还原代谢调节疗法(RMRT)通过活性氧(ROS)介导的氧化还原稳态破坏来靶向病理细胞,已成为一种有前途的无抗生素治疗方法,具有广谱疗效和降低耐药潜力。在这项研究中,我们令人惊讶地发现,替拉帕嗪(TPZ),一种临床缺氧激活的抗癌前药,通过多模式代谢干扰表现出强大的细菌诱导铁中毒能力,这表明其在肺炎治疗中的潜在用途。为了克服肺输送限制并提高治疗效果,我们设计了一种自我增强的RMRT放大器,该放大器是通过将重新利用的TPZ与天然声敏剂purpurin 18 (P18)超分子共组装而成的。超声触发的P18不仅会产生杀菌ROS,还会加剧感染部位的缺氧,从而激活TPZ,通过双重机制启动铁死亡级联:通过缺氧特异性生物激活ROS过量产生和细胞外Fe2+内流增强。值得注意的是,由此产生的嗜铁细菌作为内源性免疫刺激剂,随后引发一系列免疫反应,以建立有利于抗菌素的微环境。这种RMRT纳米放大器提供了一种安全、有效和易于获取的策略,可以协同铁中毒相关的代谢调节和免疫激活来对抗耐多药细菌性肺炎。本文章由计算机程序翻译,如有差异,请以英文原文为准。
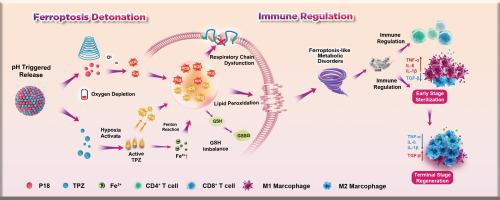
Sono-triggered nanoamplifier mediates ferroptosis-immune regulation against bacterial pneumonia
In the post-pandemic era, multidrug-resistant (MDR) bacterial pneumonia has become a critical global health challenge due to its frequent evasion of conventional antibiotic therapies. Redox metabolic regulation therapy (RMRT), which targets pathological cells through reactive oxygen species (ROS)-mediated disruption of redox homeostasis, has emerged as a promising antibiotic-free approach with broad-spectrum efficacy and reduced resistance potential. In this study, we surprisingly discovered that tirapazamine (TPZ), a clinical hypoxia-activated anticancer prodrug, exhibits potent bacterial ferroptosis-inducing capability via multimodal metabolic interference, suggesting its repurposing potential for pneumonia treatment. To overcome pulmonary delivery limitations and enhance therapeutic performance, a self-reinforcing RMRT amplifier was engineered through supramolecular co-assembly of repurposed TPZ with natural sonosensitizer purpurin 18 (P18). Ultrasound-triggered P18 not only generates bactericidal ROS but also exacerbates infection-site hypoxia, thereby activating TPZ to initiate a ferroptosis cascade via dual mechanisms: ROS overproduction through hypoxia-specific bioactivation and extracellular Fe2+ influx potentiation. Notably, the resulting ferroptotic bacteria function as endogenous immunostimulants, subsequently trigger a cascade of immunological responses to establish an antimicrobial-favorable microenvironment. Such RMRT nanoamplifier presents a safe, efficient, and easily accessible strategy that synergizes ferroptosis-associated metabolic regulation and immune activation to combat MDR bacterial pneumonia.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


