碱性磷酸酶反应水凝胶有效管理自身免疫性眼内炎症
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
自身免疫性葡萄膜炎(AU)仍然是世界范围内视力损害和失明的主要原因,需要有效和创新的治疗策略来克服当前临床干预措施的局限性。利用AU发病过程中眼内组织(玻璃体、视网膜)碱性磷酸酶(ALP)水平的显著升高,我们采用离子交联的方法合理设计了一种对ALP敏感的自我递送地塞米松磷酸钠(DexP)水凝胶。这种DexP水凝胶作为阿达木单抗(ADA)的缓释储藏库,在温和条件下,通过DexP和ADA水溶液与氯化钙的简单组合,实现AU的协同治疗,从而促进两种治疗药物以alp反应的方式控制释放。重要的是,体内实验表明,单次玻璃体内(IVT)注射ADA@DexP水凝胶通过有效抑制炎症细胞浸润、抑制促炎细胞因子(IL-17 a、IL-1β、TNF-α)和保持血视网膜屏障(BRB)的完整性,显著减轻自身免疫性眼内炎症。此外,ADA@DexP水凝胶玻璃体内给药表现出良好的眼内生物相容性和安全性。总的来说,这种无载体ADA@DexP水凝胶代表了一种有希望的AU治疗替代策略,特别是考虑到DexP和ADA都是fda批准的临床药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
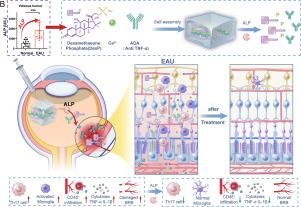

Alkaline phosphatase-responsive hydrogel for efficient management of autoimmune intraocular inflammation
Autoimmune uveitis (AU) remains a leading cause of visual impairment and blindness worldwide, necessitating efficient and innovative therapeutic strategies to overcome the limitations of current clinical interventions. Capitalizing on the significant elevation of alkaline phosphatase (ALP) levels in intraocular tissues (vitreous humor, retina) during AU pathogenesis, we rationally designed an ALP-responsive self-delivering dexamethasone sodium phosphate (DexP) hydrogel using an ionic cross-linking approach. This DexP hydrogel serves as a sustained-release depot for adalimumab (ADA), enabling synergetic AU therapy through a simple combination of aqueous DexP and ADA solutions with calcium chloride under mild conditions, which facilitates controlled release of both therapeutics in an ALP-responsive manner. Importantly, in vivo experiments demonstrated that a single intravitreal (IVT) injection of ADA@DexP hydrogel significantly attenuated autoimmune intraocular inflammation by effectively inhibiting inflammatory cell infiltration, suppressing pro-inflammatory cytokines (IL-17A, IL-1β, TNF-α), and preserving blood-retinal barrier (BRB) integrity. Furthermore, the intravitreal administration of ADA@DexP hydrogel exhibited excellent intraocular biocompatibility and a safety profile. Overall, this carrier-free ADA@DexP hydrogel represents a promising alternative strategy for AU therapy, particularly given that both DexP and ADA are FDA-approved clinical agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


