具有所有碳连接体的二聚愈创木酚内酯作为潜在的抗肝癌药物的发现
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
HCC是人类主要的恶性肿瘤,迫切需要发现新的抗HCC药物。本研究通过双- diels - alder反应,设计合成了25种全碳连接的二聚愈创木酚内酯。结果表明,化合物17的IC50值分别为1.5 μM (HepG2)、1.1 μM (hu -7)和1.1 μM (SK-Hep-1),分别比索拉非尼高4.7倍、5.5倍和8.0倍,是三种肝癌细胞增殖抑制效果最好的二聚体。生物信息学分析预测CDC45是化合物17的潜在靶点,CETSA、DARTS和SPR实验证实了这一点。化合物17以CDC45依赖的方式发挥其抗增殖作用,CDC45敲低和过表达HCC细胞,促进CDC45核输出,阻滞细胞周期在G2/M期,诱导细胞凋亡,抑制Huh-7和SK-Hep-1细胞的迁移和侵袭。体内实验表明,化合物17在30和60 mg/kg时对肿瘤重量的抑制作用分别达到76%和84%,且对主要脏器无毒性作用,不损害肝肾功能。本研究提示化合物17是一种潜在的靶向CDC45的抗肝癌药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
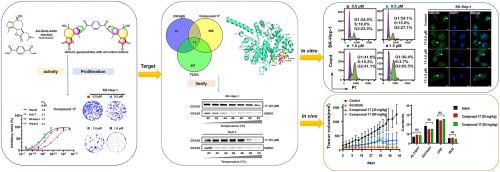
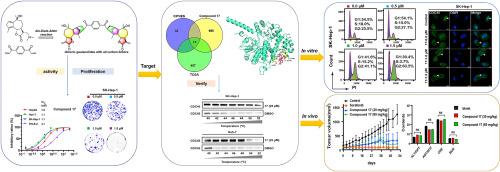
Discovery of dimeric guaianolides with all carbon linkers as potential antihepatoma agents
HCC is a major malignancy in humans, and the discovery of new anti-HCC agents is urgently needed. In this study, 25 dimeric guaianolides with all carbon linkers were designed and synthesized via a bis-Diels-Alder reaction. An evaluation of the inhibitory effects of these dimers on the proliferation of three hepatoma cell lines indicated that compound 17 was the most active dimer with IC50 values of 1.5 (HepG2), 1.1 (Huh-7), and 1.1 μM (SK-Hep-1), which were 4.7-, 5.5- and 8.0-fold more active than sorafenib, respectively. Bioinformatic analysis predicted CDC45 as a potential target of compound 17, which was confirmed by CETSA, DARTS, and SPR assays. Compound 17 exerted its antiproliferative effect in a CDC45-dependent manner as demonstrated in CDC45 knockdown and overexpression HCC cells, promoted CDC45 nuclear export, arrested the cell cycle at the G2/M phase, induced apoptosis, and inhibited the migration and invasion of Huh-7 and SK-Hep-1 cells. In vivo experiments showed that compound 17 at 30 and 60 mg/kg inhibited tumor weight up to 76 % and 84 % without causing toxicity to major organs or impairing liver and kidney function. This study suggested compound 17 as a potential antihepatoma therapeutic agent targeting CDC45.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


