克服EGFR突变抵抗:TKIs在非小细胞肺癌治疗中的双重抑制策略
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
表皮生长因子受体(EGFR)是开发新型抗癌疗法的关键靶点,特别是对于非小细胞肺癌(NSCLC)。目前,第三代EGFR抑制剂,如奥西替尼,是EGFR突变型NSCLC临床治疗的前沿。然而,由于EGFR突变和其他致癌途径驱动的耐药性的出现,它们的治疗效果已显著降低。考虑到EGFR与其他致癌途径(包括MET、HER2、VEGFR-2和PI3K)之间复杂的相互作用,双靶点抑制剂已经成为一种有希望的策略,通过同时靶向参与肿瘤生长和生存的多种途径来克服现有疗法的局限性。双靶点EGFR抑制剂的开发具有几个优点,包括改善治疗效果、减少剂量要求、降低毒性和降低耐药性发生的可能性。在这篇综述中,我们强调合理选择靶点组合,探索关键支架设计,研究特定的化学结构如何影响其作为双靶点抑制剂的生物活性。双靶点抑制剂的进展代表了非小细胞肺癌治疗的一种变革性方法,提供了一种更有效和持久的解决方案来对抗耐药和改善临床结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。

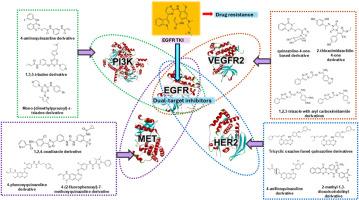
Overcoming EGFR mutation resistance: Dual inhibition strategies using TKIs in non-small cell lung cancer therapy
Epidermal Growth Factor Receptor (EGFR) is a critical target in the development of novel anticancer therapies, particularly for non-small cell lung cancer (NSCLC). Currently, third-generation EGFR inhibitors, such as osimertinib, are at the forefront of clinical treatment for EGFR-mutant NSCLC. However, their therapeutic efficacy has been significantly compromised by the emergence of drug resistance, driven by EGFR mutations and alternative oncogenic pathways. Given the complex interplay between EGFR and other oncogenic pathways, including MET, HER2, VEGFR-2, and PI3K, dual-target inhibitors have emerged as a promising strategy to overcome the limitations of existing therapies by simultaneously targeting multiple pathways involved in tumour growth and survival. The development of dual-target EGFR inhibitors offers several advantages, including improved therapeutic efficacy, reduced dosage requirements, lower toxicity, and a decreased likelihood of resistance development. In this review, we emphasize the rational selection of target combinations and explore key scaffold designs, examining how specific chemical structures influence their biological activity as dual-target inhibitors. The advancement of dual-target inhibitors represents a transformative approach to NSCLC treatment, offering a more effective and durable solution to combat drug resistance and improve clinical outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


