可生物降解纳米纤维支架通过局部靶向治疗提高胶质母细胞瘤的护理标准
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
联合治疗是治疗侵袭性癌症的一种行之有效的临床策略,但其成功尚未转化为多形性胶质母细胞瘤(GBM)患者-中枢神经系统最具侵袭性的恶性肿瘤。在这项研究中,我们评估了替莫唑胺(TMZ) (GBM的标准化疗药物)与几种候选靶向治疗联合使用的效果,以改善GBM切除和复发的小鼠模型的当前结果。在体外,EGFR抑制剂厄洛替尼(ERL)在多种GBM细胞中成为最有希望的联合药物。在体内,通过局部给药,治疗反应得到增强。ERL被封装到静电纺醋酸化葡聚糖(Ace-DEX)支架(Ace-ERL)中,这是一种可生物降解和生物相容性的聚合物系统,可以调节降解和控制药物释放。在患者来源的异种移植小鼠模型中,Ace-ERL局部递送到切除腔改善了药代动力学特征,并且当与全身性TMZ联合使用时,显著提高了生存率。这些发现支持一种新的转化方法,通过将靶向给药与标准化疗相结合来利用GBM的联合治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
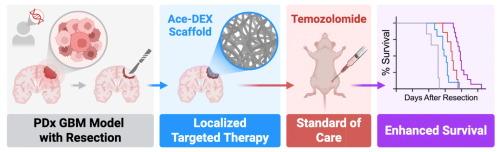
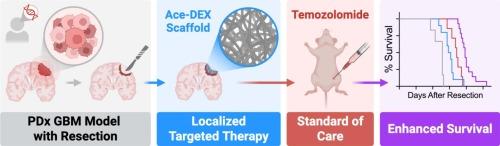
Biodegradable nanofibrous scaffolds enhance standard of care for glioblastoma via localized targeted therapy
Combination therapy is a well-established clinical strategy for treating aggressive cancers, but its success has not translated to patients with glioblastoma multiforme (GBM)—the most aggressive malignancy of the central nervous system. In this study, we evaluated the effects of combining temozolomide (TMZ), the standard chemotherapeutic agent for GBM, with several candidate targeted therapies to improve current outcomes in a mouse model of GBM resection and recurrence. In vitro, the EGFR inhibitor, erlotinib (ERL), emerged as the most promising combination drug across a diverse panel of GBM cells. In vivo, the therapeutic response was enhanced through localized delivery. ERL was encapsulated into electrospun acetalated dextran (Ace-DEX) scaffolds (Ace-ERL), a biodegradable and biocompatible polymer system that enables tunable degradation and controlled drug release. Local delivery of Ace-ERL to the resection cavity improved the pharmacokinetic profile and, when combined with systemic TMZ, significantly enhanced survival in a patient-derived xenograft mouse model. These findings support a novel translational approach to leverage combination therapy in GBM by pairing targeted delivery with standard chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


