双机制黏附水凝胶整合ph非依赖性屏障和自噬驱动免疫疗法治疗溃疡性结肠炎。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
目前溃疡性结肠炎(UC)的治疗面临着严峻的挑战,包括全身毒性和粘膜再生不足。在此,我们提出了一个开创性的双机制治疗平台,将ph不依赖酸处理的硫糖铝(ASF)与诱导自噬的亚精胺(Spd)结合起来,协同治疗UC的发病机制。与传统的ph依赖性屏障疗法不同,ASF形成一种机械可调、可注射的水凝胶,无论局部ph如何,它都能牢固地粘附在结肠黏膜上。亚精胺通过静电相互作用很容易地装入ASF水凝胶基质,包封效率超过90%。此外,通过调整配方中亚精胺的含量,可以精确调节ASF水凝胶的机械强度,使其适合于有效覆盖肠道黏膜。重要的是,体外渗透性试验表明,载亚精胺的ASF水凝胶(Spd-ASF)具有选择性屏障功能,可阻断80%的大肠杆菌和LPS的渗透,同时允许营养物质渗透。此外,显微ct图像显示,直肠输注Spd-ASF水凝胶在结肠壁上均匀粘附至少8小时。在dss诱导的结肠炎小鼠中,直肠输注Spd-ASF水凝胶使结肠长度恢复到接近正常水平(对照组缩短30%),促炎细胞因子(TNF-α, IL-6, IL-1β)减少60-75%,杯状细胞密度增加一倍。在机制上,Spd-ASF通过激活自噬和增强efferocytosis,驱动M2极化来解决炎症,从而重新编程巨噬细胞的行为。值得注意的是,Spd-ASF独特地逆转了生态失调,提高了有益的乳酸杆菌,同时抑制了与结肠炎相关的Muribaculaceae和Clostridia。这项研究首次将黏合剂生物材料工程与自噬介导的免疫调节相结合,通过同时屏蔽粘膜和重编程炎症途径,为UC治疗提供了范式转变。意义声明:本研究将诱导自噬的亚精胺(Spd)和不依赖ph的酸处理的硫糖铝(ASF)结合在一起,形成了一种新的双机制水凝胶(Spd-ASF),它可以协同对抗UC的发病机制。Spd-ASF水凝胶的选择性屏障功能是通过其允许营养渗透的能力而建立的,同时阻止毒素(LPS)和致病菌(大肠杆菌)的转移。此外,微ct图像显示,直肠注入的Spd-ASF水凝胶在结肠壁上持续附着至少8小时。Spd-ASF治疗可使杯状细胞密度增加一倍,使促炎细胞因子(TNF-α、IL-6和IL-1β)降低60-75%,促进有益乳杆菌的生长,降低致病性Muribaculaceae和Clostridia。它还能恢复dss诱导的结肠炎动物的结肠长度。Spd-ASF的治疗作用与促进细胞efferocytosis和触发巨噬细胞自噬密切相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
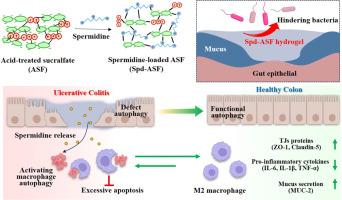
Dual-mechanism mucoadhesive hydrogel integrating pH-independent barriers and autophagy-driven immunotherapy for the treatment of ulcerative colitis
Current therapies for ulcerative colitis (UC) face critical challenges, including systemic toxicity and inadequate mucosal regeneration. Herein, we present a pioneering dual-mechanism therapeutic platform integrating pH-independent acid-treated sucralfate (ASF) with autophagy-inducing spermidine (Spd) to synergistically address UC pathogenesis. Unlike conventional pH-dependent barrier therapies, ASF forms a mechanically tunable, injectable hydrogel that adheres robustly to colonic mucosa regardless of local pH. Spermidine was easily mounted into ASF hydrogel matrix via electrostatic interaction, with more than 90 % encapsulation efficiency. Moreover, the mechanical strength of ASF hydrogel was precisely modulated by adjusting spermidine amount in formula, making it suitable for effective gut mucosa coverage. Importantly, in vitro permeability test showed that spermidine-loaded ASF hydrogel (Spd-ASF) demonstrated the selective barrier functionality, blocking > 80 % of Escherichia coli and LPS penetration while allowing nutrient permeability. Moreover, micro-CT images demonstrated that rectally infused Spd-ASF hydrogel was uniformly adhered to the colon wall at least for 8 h. In DSS-induced colitis mice, rectally administrating Spd-ASF hydrogel uniquely restored colon length to near-normal levels (vs. 30 % shortening in controls), reduced proinflammatory cytokines (TNF-α, IL-6, IL-1β) by 60–75 %, and doubled goblet cell density. Mechanistically, Spd-ASF reprogrammed macrophage behavior by activating autophagy and enhancing efferocytosis, driving M2 polarization to resolve inflammation. Notably, Spd-ASF uniquely reversed dysbiosis, elevating beneficial Lactobacillus while suppressing colitis-associated Muribaculaceae and Clostridia. This study is the first to combine mucoadhesive biomaterial engineering with autophagy-mediated immunomodulation, offering a paradigm shift in UC therapy by simultaneously shielding the mucosa and reprogramming inflammatory pathways.
Statement of significance
Here, autophagy-inducing spermidine (Spd) and pH-independent acid-treated sucralfate (ASF) were combined to create the novel dual-mechanism hydrogel (Spd-ASF), which works in concert to combat UC pathogenesis. The selective barrier functioning of Spd-ASF hydrogel was established by its capacity to permit nutritional permeability while preventing the transfer of toxins (LPS) and pathogenic bacteria (E. coli). Additionally, rectally infused Spd-ASF hydrogel was consistently attached on the colon wall for at least eight hours, as seen by micro-CT images. Spd-ASF therapy doubled the density of goblet cells, decreased proinflammatory cytokines (TNF-α, IL-6, and IL-1β) by 60–75 %, boosted helpful Lactobacillus, and lowered pathogenic Muribaculaceae and Clostridia. It also restored colon length in DSS-induced colitis animals. Spd-ASF's therapeutic action was closely linked to promoting cellular efferocytosis and triggering macrophage autophagy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


