水合Meranzin通过恢复肠道微生物群驱动的微生物炎症通路改善ApoE-/-小鼠的“动脉粥样硬化抑制”
IF 2.5
3区 医学
Q2 BEHAVIORAL SCIENCES
引用次数: 0
摘要
背景:抑郁症和动脉粥样硬化经常同时发生,肠道菌群驱动的炎症回路被破坏是一种共同的致病机制。为了解决这个问题,我们将“动脉粥样硬化性抑郁症”定义为抑郁症与动脉粥样硬化的合并症。尽管具有临床意义,但仍缺乏综合治疗策略。方法:建立ApoE-/-高脂饮食与急性约束应激相结合的小鼠模型,模拟动脉粥样硬化相关性抑郁。动物接受水合Meranzin (MH)治疗,然后进行综合评估。代谢和血管终点包括体重、血脂和主动脉斑块负荷(油红O染色)。抑郁样行为通过标准化行为测试进行评估。BOLD-fMRI结合区域均匀性(ReHo)分析测量脑功能活动。通过16S rRNA测序进行肠道微生物群分析,评估α-/β-多样性、厚壁菌门/拟杆菌门比例和关键分类群。ELISA检测炎症和神经营养标志物(5-羟色胺、脑源性神经营养因子、白细胞介素-6、内皮型一氧化氮合酶和胃饥饿素),Western blot和RT-PCR检测血管细胞粘附分子-1、白细胞介素-1β、肿瘤坏死因子-α和胃饥饿素的蛋白和mRNA水平。结果:MH显著减少主动脉斑块形成,改善血脂,减轻抑郁样行为。MH通过增强α-多样性,使厚壁菌门/拟杆菌门比例正常化,丰富有益类群如Akkermansia和Allobaculum等,恢复肠道微生物群的稳态。这些微生物群的变化与应激和饮食诱导的情绪调节区域(海马体和前额皮质)BOLD-fMRI改变的逆转是平行的。炎症回路被重新平衡,IL-6、TNF-α、IL-1β和VCAM-1的降低,以及海马和心脏5-HT、BDNF和胃饥饿素水平的恢复证明了这一点。结论:MH通过调节急性精神应激诱导的血管炎症、神经营养因子、脑功能和肠道微生物群,具有缓解动脉粥样硬化共抑郁的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
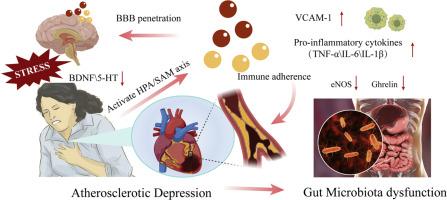
Meranzin Hydrate ameliorates “atherosclerotic depression” in ApoE−/− mice by restoring gut microbiota—driven microbial-inflammatory circuitry
Background
Depression and atherosclerosis frequently co-occur, with disrupted gut microbiota–driven inflammatory circuitry emerging as a shared pathogenic mechanism. To address this, we operationally define “atherosclerotic depression” as depression comorbidity with atherosclerosis. Despite its clinical significance, integrated therapeutic strategies remain lacking.
Methods
An ApoE−/− mouse model combining high-fat diet feeding and acute restraint stress was established to mimic atherosclerosis-associated depression. Animals received Meranzin Hydrate (MH) treatment, followed by comprehensive assessments. Metabolic and vascular endpoints included body weight, serum lipid profiles, and aortic plaque burden (Oil Red O staining). Depressive-like behaviors were evaluated through standardized behavioral tests. BOLD-fMRI with regional homogeneity (ReHo) analysis measured brain functional activity. Gut microbiota profiling was performed by 16S rRNA sequencing to assess α-/β-diversity, Firmicutes/Bacteroidetes ratio, and keystone taxa. Inflammatory and neurotrophic markers were examined via ELISA (5-hydroxytryptamine, brain-derived neurotrophic factor, interleukin-6, endothelial nitric oxide synthase, and ghrelin), while protein and mRNA levels of vascular cell adhesion molecule-1, interleukin-1β, tumor necrosis factor-α, and ghrelin were analyzed by Western blot and RT-PCR.
Results
MH significantly reduced aortic plaque formation, improved lipid profiles, and alleviated depressive-like behaviors. MH restored gut microbiota homeostasis by enhancing α-diversity, normalizing the Firmicutes/Bacteroidetes ratio, and enriching beneficial taxa such as Akkermansia and Allobaculum. These microbiota changes paralleled the reversal of stress- and diet-induced BOLD-fMRI alterations in emotion-regulation regions (hippocampus and prefrontal cortex). Inflammatory circuitry was rebalanced, as evidenced by reduced IL-6, TNF-α, IL-1β, and VCAM-1, alongside restored hippocampal and cardiac 5-HT, BDNF, and ghrelin levels.
Conclusion
MH demonstrates therapeutic potential in alleviating atherosclerosis co-depression by modulating acute mental stress-induced vascular inflammation, neurotrophic factors, brain function, and gut microbiota.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physiology & Behavior
医学-行为科学
CiteScore
5.70
自引率
3.40%
发文量
274
审稿时长
47 days
期刊介绍:
Physiology & Behavior is aimed at the causal physiological mechanisms of behavior and its modulation by environmental factors. The journal invites original reports in the broad area of behavioral and cognitive neuroscience, in which at least one variable is physiological and the primary emphasis and theoretical context are behavioral. The range of subjects includes behavioral neuroendocrinology, psychoneuroimmunology, learning and memory, ingestion, social behavior, and studies related to the mechanisms of psychopathology. Contemporary reviews and theoretical articles are welcomed and the Editors invite such proposals from interested authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


