Batatasin III通过调节肠道菌群和抑制NLRP3-IL-1β途径缓解慢传输型便秘
IF 4.9
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
背景:慢传输型便秘(STC)是一种以肠道动力受损、炎症反应和肠道菌群失调为特征的功能性胃肠疾病。Batatasin III是一种天然二苯乙烯化合物,已被证明具有抗炎和神经保护特性;然而,它在STC中的作用尚不清楚。方法:建立洛哌丁胺致STC小鼠模型,分别给予低剂量和高剂量Batatasin III治疗。通过一般表型观察、小肠转运率测定、苏木精-伊红(HE)染色、酶联免疫吸附试验(ELISA)、定量逆转录聚合酶链反应(qRT-PCR)、Western blotting、免疫荧光、16S rRNA测序等方法评价治疗效果。此外,通过Spearman相关分析研究肠道菌群与炎症细胞因子和神经递质之间的关系。结果:Batatasin III显著改善小鼠粪便参数和肠道转运率,恢复结肠组织结构,调节5-羟色胺(5-HT)和P物质(SP)等神经递质水平。此外,它还能抑制NLRP3-IL-1β信号通路的激活,降低促炎细胞因子的表达。肠道菌群测序显示,有益细菌如拟副杆菌和Faecalibaculum增加,同时促炎Rikenellaceae的丰度减少。这些微生物变化与炎症标志物和神经递质水平显著相关。结论:Batatasin III通过调节肠道菌群和抑制NLRP3-IL-1β炎症通路缓解stc相关症状。其治疗作用可能通过微生物-肠-脑轴(MGBA)介导,突出了其作为治疗慢传输型便秘的新型治疗剂的潜在价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
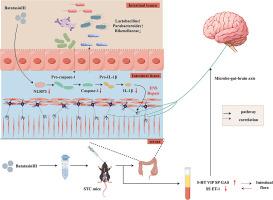
Batatasin III alleviates slow transit constipation by regulating gut microbiota and inhibiting the NLRP3-IL-1β pathway
Slow transit constipation (STC) is a functional gastrointestinal disorder characterized by impaired intestinal motility, inflammatory responses, and gut microbiota dysbiosis. Batatasin III, a natural stilbene compound, has been demonstrated to exhibit anti-inflammatory and neuroprotective properties; however, its role in STC remains unclear. In this study, a loperamide-induced STC mouse model was established and treated with low and high doses of Batatasin III. The therapeutic effects were evaluated through general phenotypic observation, small intestinal transit rate measurement, hematoxylin-eosin (HE) staining, enzyme-linked immunosorbent assay (ELISA), quantitative reverse transcription polymerase chain reaction (qRT-PCR), Western blotting, immunofluorescence, and 16S rRNA sequencing. Furthermore, Spearman correlation analysis was conducted to investigate the relationships between the gut microbiota and inflammatory cytokines and neurotransmitters. Batatasin III significantly improved fecal parameters and intestinal transit rate in mice, restored colonic tissue structure, and modulated levels of neurotransmitters such as 5-hydroxytryptamine (5-HT) and substance P (SP). Additionally, it inhibited activation of the NLRP3–IL-1β signaling pathway and reduced the expression of pro-inflammatory cytokines. Gut microbiota sequencing revealed an increase in beneficial bacteria such as Parabacteroides and Faecalibaculum, alongside a decreased abundance of the pro-inflammatory Rikenellaceae. These microbial changes were significantly correlated with both inflammatory markers and neurotransmitter levels. Batatasin III alleviates STC-related symptoms by modulating the gut microbiota and inhibiting the NLRP3–IL-1β inflammatory pathway. Its therapeutic effects may be mediated through the microbiota–gut–brain axis (MGBA), highlighting its potential value as a novel therapeutic agent for the treatment of slow transit constipation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nutritional Biochemistry
医学-生化与分子生物学
CiteScore
9.50
自引率
3.60%
发文量
237
审稿时长
68 days
期刊介绍:
Devoted to advancements in nutritional sciences, The Journal of Nutritional Biochemistry presents experimental nutrition research as it relates to: biochemistry, molecular biology, toxicology, or physiology.
Rigorous reviews by an international editorial board of distinguished scientists ensure publication of the most current and key research being conducted in nutrition at the cellular, animal and human level. In addition to its monthly features of critical reviews and research articles, The Journal of Nutritional Biochemistry also periodically publishes emerging issues, experimental methods, and other types of articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


