光诱导不饱和酰胺与硫氰酸酯串联环化以获得硫氰化杂环
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
建立了光诱导的n -丙烯醛腙/不饱和香豆素与硫氰酸酯的两个自由基硫氰化环化体系,该体系高效地合成了多种硫氰化吡唑酮/多环香豆素,并具有良好的选择性。同时,各种控制实验(放大操作、取代基转化、自由基捕获/开-关操作)探索了硫氰化体系的优点和合理的环化过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
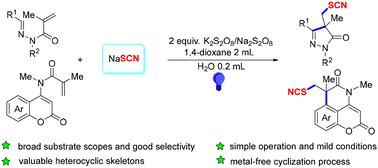
Photo-induced tandem cyclization of unsaturated amides with thiocyanates to access thiocyanated heterocycles
Photo-induced two-radical thiocyanation cyclization systems of N-acryloyl aldehyde hydrazones/unsaturated coumarins with thiocyanate were completed, efficiently producing diverse thiocyanated pyrazolones/polycyclic coumarins with good selectivity. Meanwhile, various control experiments (scaled-up operations, substituent transformations, and radical capture/light-on–off operations) explored the merits and the plausible cyclization process of the thiocyanation systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


