elavl1稳定的USP22通过触发ACSL4去泛素化介导足细胞损伤和死亡,从而促进糖尿病肾病的进展。
IF 1.4
4区 医学
Q4 IMMUNOLOGY
引用次数: 0
摘要
背景:糖尿病肾病(DN)约占所有慢性肾病病例的50% %。鉴于USP22参与DN的进展,本研究探讨了其潜在的调控机制。方法:采用高糖处理小鼠足细胞,建立糖尿病小鼠模型。CCK-8和TUNEL/流式细胞术分别检测足细胞活力和凋亡。根据制造商的协议,使用ELISA和商业试剂盒对铁凋亡标志物(Fe2+、ROS、MDA和GSH)和炎症细胞因子进行定量。通过蛋白稳定性和共免疫沉淀(Co-IP)实验证实了USP22与ACSL4的相互作用。此外,采用RNA免疫沉淀(RIP)和mRNA稳定性分析来阐明ELAVL1/USP22的相互作用。结果:在HG-treated足细胞,USP22沉默增强细胞活力(P = 0.0018),压抑的细胞凋亡(P = 0.0019),并降低炎性细胞因子的释放(il - 1β:P = 0.0002;肿瘤坏死因子-α:P 2 +:P = 0.0002;活性氧:P = 0.0005;MDA: P = 0.0017;谷胱甘肽:P = 0.0086)。相反,在hg处理的足细胞中,USP22过表达表现出相反的作用(P 结论:elavl1稳定的USP22通过ACSL4去泛素化依赖机制加重足细胞损伤,增强炎症反应和细胞死亡,从而促进DN的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
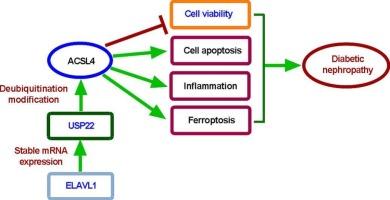
ELAVL1-stabilized USP22 promotes diabetic nephropathy progression via mediating podocyte injury and death by triggering ACSL4 deubiquitination
Background
Diabetic nephropathy (DN) represents approximately 50 % of all chronic kidney disease cases. Given the established involvement of USP22 in DN progression, this study investigated its underlying regulatory mechanisms.
Methods
Mouse podocytes were treated with high glucose (HG), and a diabetic mouse model was established. Podocyte viability and apoptosis were assessed by CCK-8 and TUNEL/flow cytometry, respectively. Ferroptosis markers (Fe2+, ROS, MDA, and GSH) and inflammatory cytokines were quantified using ELISA and commercial kits per manufacturers' protocols. The interaction of USP22 with ACSL4 was demonstrated through protein stability and co-immunoprecipitation (Co-IP) assays. Additionally, RNA immunoprecipitation (RIP) and mRNA stability assays were employed to elucidate the ELAVL1/USP22 interaction.
Results
In HG-treated podocytes, USP22 silencing enhanced cell viability (P = 0.0018), repressed apoptosis (P = 0.0019), and reduced the release of inflammatory cytokines (IL-1β: P = 0.0002; TNF-α: P < 0.0001) and ferroptosis markers (Fe2+: P = 0.0002; ROS: P = 0.0005; MDA: P = 0.0017; GSH: P = 0.0086). Conversely, USP22 overexpression in HG-treated podocytes exhibited the opposite effects (P < 0.05). USP22 increased ACSL4 expression (P = 0.0012) in a deubiquitination-dependent manner. Notably, ACSL4 overexpression rescued USP22 depletion-mediated alterations on cell viability, apoptosis, inflammation, and ferroptosis (P < 0.05). Moreover, ELAVL1 stabilized USP22 mRNA through interaction (P = 0.0075). USP22 silencing alleviated DN progression and reduced inflammation cytokine secretion in a diabetic mouse model (P < 0.05).
Conclusion
ELAVL1-stabilized USP22 promotes DN progression by exacerbating podocyte injury and enhancing inflammatory responses and cell death through ACSL4 deubiquitination-dependent mechanisms.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Transplant immunology
医学-免疫学
CiteScore
2.10
自引率
13.30%
发文量
198
审稿时长
48 days
期刊介绍:
Transplant Immunology will publish up-to-date information on all aspects of the broad field it encompasses. The journal will be directed at (basic) scientists, tissue typers, transplant physicians and surgeons, and research and data on all immunological aspects of organ-, tissue- and (haematopoietic) stem cell transplantation are of potential interest to the readers of Transplant Immunology. Original papers, Review articles and Hypotheses will be considered for publication and submitted manuscripts will be rapidly peer-reviewed and published. They will be judged on the basis of scientific merit, originality, timeliness and quality.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


