Esculetin通过创新的多靶点抑制金黄色葡萄球菌分类酶A、凝固酶和血管性血友病因子结合蛋白来减轻MRSA的致病性。
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-09-08
DOI:10.1016/j.ijantimicag.2025.107609
引用次数: 0
摘要
耐抗生素金黄色葡萄球菌,特别是耐甲氧西林金黄色葡萄球菌(MRSA)的全球扩散,突出了迫切需要创新的抗毒策略。金黄色葡萄球菌产生的毒力因子的冗余性和多样性需要能够同时针对多种毒力机制的干预措施。在本研究中,我们通过化合物文库的综合筛选,鉴定了天然化合物esculetin。Esculetin独特地靶向分选酶A (SrtA)、凝固酶(Coa)和血管性血友病因子结合蛋白(vWbp),而不抑制细菌的体外生长。Esculetin显著减弱srta介导的MRSA USA300菌株的侵袭和生物膜的形成,同时抑制vWbp和Coa的凝固酶活性。通过荧光共振能量转移(FRET)、荧光猝灭和热移分析,我们证实了esculetin与SrtA、Coa和vWbp之间的直接结合相互作用。体内研究表明,esculetin显著降低了mellonella幼虫的MRSA毒力。值得注意的是,与万古霉素单药治疗相比,埃斯库霉素联合万古霉素可显著增强小鼠模型对MRSA usa300诱导的肺炎和皮肤感染的治疗效果,具有更好的保护作用。本研究首次全面展示了一种能够同时抑制金黄色葡萄球菌多种毒力因子的天然化合物。esculetin的多靶点抑制策略通过增强现有抗生素的治疗潜力和降低耐药性发展的可能性,代表了对抗耐药金黄色葡萄球菌感染的一个有希望的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
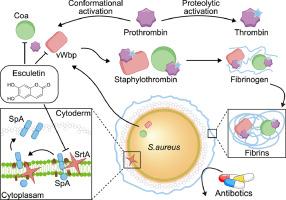
Esculetin mitigates MRSA pathogenicity via innovative multitarget inhibition of staphylococcus aureus sortase a, coagulase, and von Willebrand factor-binding protein
The global proliferation of antibiotic-resistant Staphylococcus aureus, particularly methicillin-resistant Staphylococcus aureus (MRSA), highlights the urgent need for innovative antivirulence strategies. The redundancy and multiplicity of virulence factors produced by S. aureus necessitate interventions capable of concurrently targeting multiple virulence mechanisms. In this study, we identified esculetin, a natural compound, through comprehensive screening of a compound library. Esculetin uniquely targets sortase A (SrtA), coagulase (Coa), and von Willebrand factor-binding protein (vWbp) without inhibiting bacterial growth in vitro. Esculetin significantly attenuated SrtA-mediated MRSA USA300 strain invasion and biofilm formation while also inhibiting the coagulase activities of both vWbp and Coa. Using fluorescence resonance energy transfer (FRET), fluorescence quenching, and thermal shift assays, we confirmed direct binding interactions between esculetin and SrtA, Coa, and vWbp. In vivo studies demonstrated that esculetin significantly reduced MRSA virulence in Galleria mellonella larvae. Notably, the combination of esculetin and vancomycin markedly enhanced the therapeutic efficacy against MRSA USA300-induced pneumonia and skin infections in murine models, providing superior protection compared with vancomycin monotherapy. This study presents the first comprehensive demonstration of a natural compound capable of simultaneously inhibiting multiple virulence factors of S. aureus. The multitarget inhibition strategy of esculetin represents a promising advancement in combating antibiotic-resistant S. aureus infections by enhancing the therapeutic potential of existing antibiotics and reducing the likelihood of resistance development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


