铜催化亚砜胺对映特异性芳化合成手性亚砜胺
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
手性亚酰基亚胺是一类手性S(IV)化合物,可作为手性S(VI)化合物的药效载体和手性组分。在此,我们报告了一种有效的方法,从市售的二芳硫鎓盐和前手性亚砜胺制备手性亚砜胺(高达90%的产率和高达96%的对映体过剩)。该反应被放大到克级,以制备手性亚砜亚胺,并允许手性亚砜亚胺和磺基二胺的衍生化,并完全保留立体化学。本文章由计算机程序翻译,如有差异,请以英文原文为准。

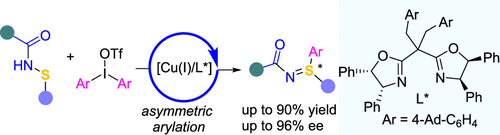
Synthesis of Chiral Sulfilimines via Copper-Catalyzed Enantiospecific Arylation of Sulfenamides
Chiral sulfilimines represent a type of chiral S(IV) compound that is versatile as pharmacophores and chiral building blocks for chiral S(VI) compounds. Herein, we report an efficient method of preparing chiral sulfilimines (up to 90% yield and up to 96% enantiomeric excess) from commercially available diaryliodonium salts and prochiral sulfenamides. The reaction was enlarged to the gram scale for the preparation of chiral sulfilimines and allowed for the derivatization of chiral sulfoximines and sulfondiimines with complete retention of stereochemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


