喹啉-2(1H)- 1与氟烷基溴的EDA配合物引发C-H二氟烷基化
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过喹啉-2(1H)- 1与氟烷基溴的C-H二氟烷基化,建立了一种有效的可见光诱导EDA策略,构建了3-二氟烷基化喹啉-2(1H)- 1。该转化可以在温和和无金属的条件下,获得一系列中高产率的3-二氟烷基化喹啉-2(1H)-。机理研究表明,氟烷基溴和TMEDA之间EDA络合物的形成对于启动这一转变至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。

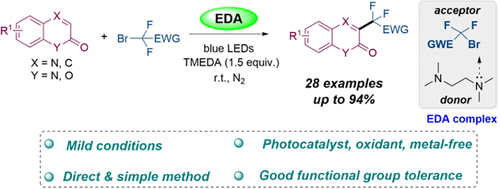
EDA Complex Initiated C–H Difluoroalkylation of Quinoxalin-2(1H)-ones with Fluoroalkyl Bromides
An efficient visible-light-induced EDA strategy has been developed for constructing 3-difluoroalkylated quinoxalin-2(1H)-ones through the C–H difluoroalkylation of quinoxalin-2(1H)-ones with fluoroalkyl bromides. This transformation could provide a series of 3-difluoroalkylated quinoxalin-2(1H)-ones in moderate to good yields under mild and metal-free conditions. Mechanistic studies revealed that the formation of the EDA complex between fluoroalkyl bromide and TMEDA was essential to initiate this transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


