损伤诱导的IL-18刺激胸腺NK细胞,限制内源性组织再生
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
白细胞介素-18 (IL-18)是一种急性期促炎分子,通过激活T辅助1 CD4+ T细胞、细胞毒性CD8+ T细胞和自然杀伤(NK)细胞介导病毒清除。在这里,我们发现成熟的IL-18在胸腺中产生后,许多不同形式的组织损伤,所有这些都导致caspase-1介导的免疫原性细胞死亡。我们报道il -18刺激的细胞毒性NK细胞限制内源性胸腺再生,这是确保急性损伤(如应激、感染、化疗和放疗)后免疫能力恢复的关键过程。NK细胞通过异常靶向胸腺上皮细胞抑制胸腺恢复,而胸腺上皮细胞是器官功能和再生的主要调节因子。总之,我们的数据揭示了调节胸腺组织再生的新途径,并建议IL-18作为促进胸腺功能的潜在治疗靶点。此外,鉴于IL-18作为癌症免疫疗法的热情,由于其能够引发1型免疫反应,这些发现也为潜在的脱靶效应提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
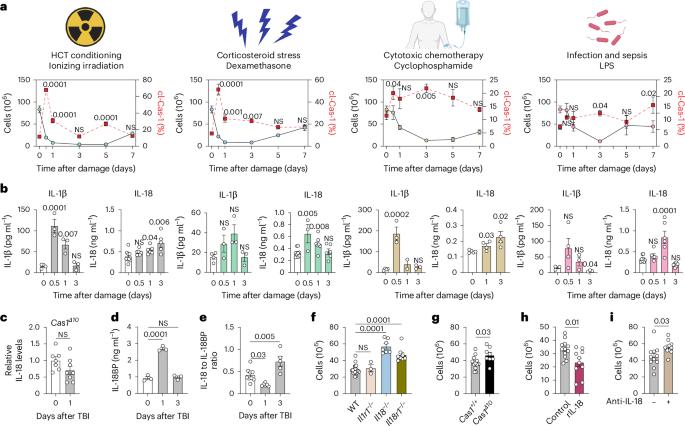
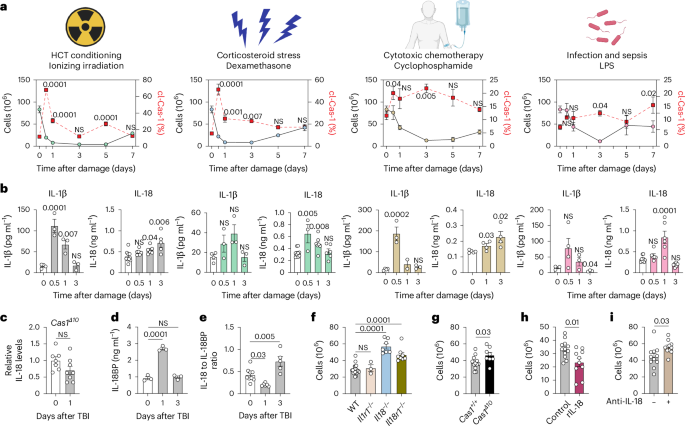
Damage-induced IL-18 stimulates thymic NK cells limiting endogenous tissue regeneration
Interleukin-18 (IL-18) is an acute-phase proinflammatory molecule crucial for mediating viral clearance by activating T helper 1 CD4+ T cells, cytotoxic CD8+ T cells and natural killer (NK) cells. Here, we show that mature IL-18 is generated in the thymus following numerous distinct forms of tissue damage, all of which cause caspase-1-mediated immunogenic cell death. We report that IL-18-stimulated cytotoxic NK cells limit endogenous thymic regeneration, a critical process that ensures the restoration of immune competence after acute insults such as stress, infection, chemotherapy and radiation. NK cells suppress thymus recovery by aberrantly targeting thymic epithelial cells, which act as the master regulators of organ function and regeneration. Together, our data reveal a new pathway regulating tissue regeneration in the thymus and suggest IL-18 as a potential therapeutic target to boost thymic function. Moreover, given the enthusiasm for IL-18 as a cancer immunotherapy due to its capacity to elicit a type 1 immune response, these findings also offer insight into potential off-target effects. The thymus is sensitive to acute insults including infection, as well as to injury from chemotherapy and myeloablative conditioning before hematopoietic cell transplantation. Here, Granadier et al. describe a role for IL-18 in limiting thymic regeneration by stimulating NK cells, which then target thymic epithelial cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


