揭开聚山梨酯20组成:UPLC-MS分析和随机建模的融合。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-07
DOI:10.1016/j.ejpb.2025.114854
引用次数: 0
摘要
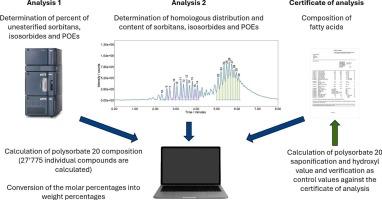
聚山梨酯20 (PS20)是化妆品、药品和食品中最常用的非离子表面活性剂之一。考虑到生物相容性和无刺激性,它的溶解和蛋白质稳定特性进一步受到重视。PS20是通过山梨醇与各种脂肪酸和环氧乙烷的多阶段反应,得到具有不同分子量和极性组分的复杂混合物。由于这些组分分布的变化会影响其性能,例如乳化或增溶效率,因此详细了解PS20的组成非常重要。在这里,我们介绍了反相色谱与质量检测和自动随机建模的组合方法,使PS20在组分水平上的定量表征成为可能。该技术采用两种简单的样品制备和两种方法,用于超高性能液相色谱(UPLC)系统,并与单个四极杆质量(QDa)检测器相结合,确保了高效的数据采集。采用该方法对不同厂家、生产年限和质量的7种PS20产品进行了研究。计算了PS20中超过27700种成分的摩尔含量和重量百分比,这些成分完全由i)物质类别(即山梨糖、异山梨脂或聚氧乙烯(POE))、ii)酯的数量、iii)脂肪酸组合和iv) OE单位的数量来表征。所得结果不仅可以准确预测体积参数,如羟基和皂化值,而且可以进行详细的产品比较。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unravelling the polysorbate 20 composition: A fusion of UPLC-MS analysis and stochastic modelling
Polysorbate 20 (PS20) is one of the most commonly used non-ionic surfactants in cosmetics, pharmaceuticals and food products. Considered as biocompatible and non-irritating, it is further valued for its solubilising and protein stabilising properties. PS20 is manufactured through a multi-stage reaction of sorbitol with various fatty acids and ethylene oxide, resulting in a complex mixture of components with different molecular weights and polarity. Since variations in the distribution of these components can influence its performance, such as the emulsifying or solubilising efficiency, a detailed understanding of the PS20 composition is of importance.
Herein we introduce a combined approach of reversed-phase chromatography with mass detection and automated stochastic modelling that enables the quantitative characterisation of PS20 at the component level. With two straightforward sample preparations and two methods for an ultra-high performance liquid chromatography (UPLC) system coupled to a single quadrupole mass (QDa) detector, this technique ensures efficient data acquisition. Seven PS20 products of different manufacturers, age and qualities were studied using the presented approach. Molar contents and weight percentages were calculated for each of the more than 27′700 components of the PS20, which were fully characterised by i) the substance class (i.e. sorbitan, isosorbide or polyoxyethylene (POE)), ii) the number of esters, iii) the fatty acid combination and iv) the number of OE units. The obtained results allowed not only an accurate prediction of bulk parameters, such as hydroxyl and saponification values, but also a detailed product comparison.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


