为前药生物等效性风险评估建立临床相关的溶出度规范:在醋酸阿比特龙中整合溶出/渗透系统和基于生理的生物制药模型。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-09-07
DOI:10.1016/j.ejpb.2025.114857
引用次数: 0
摘要
需要酶激活的前药,如弱碱性生物制药分类系统(BCS) IV类化合物醋酸阿比特酮(ABA),由于其ph依赖性溶解度、食物效应和可变的肠道水解,面临相当大的生物等效性(BE)风险。本研究利用生物相关溶出度和基于生理的生物制药模型(PBBM)建立了与临床相关的ABA溶出度规范。两种溶出方法,两阶段(胃肠道转移模拟)和单相(生物相关介质),在禁食和喂养条件下进行评估。临床BE研究显示配方A(禁食,N = 39)无BE,但配方B(禁食/喂养,N = 40)符合BE。两阶段溶解突出了过饱和动力学,与禁食条件相比,喂食状态介质(FeSSIF-V2)的溶解度提高了 >10倍。尽管这种方法具有生物预测性,但其复杂性限制了其实用性。使用生物相关介质的单相溶出平衡了鉴别能力和操作可行性,以进行质量控制。通透性研究发现,活性代谢物阿比特龙是ABA的吸收驱动因子(表观通透系数:1.55 × 10-5 vs. 8.91 × 10- 26 cm/s)。综合水解动力学、食物效应和首过代谢的PBBM预测临床药代动力学,预测误差为本文章由计算机程序翻译,如有差异,请以英文原文为准。
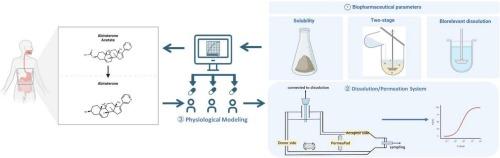
Establishing clinically relevant dissolution specifications for prodrug bioequivalence risk assessment: Integration of a dissolution/permeation system with physiologically based biopharmaceutics modeling in abiraterone acetate
Prodrugs with enzymatic activation requirements, such as the weakly basic biopharmaceutical classification system (BCS) class IV compound abiraterone acetate (ABA), face considerable bioequivalence (BE) risks owing to their pH-dependent solubility, food effects, and variable intestinal hydrolysis. This study established clinically relevant dissolution specifications for ABA using biorelevant dissolution and physiologically based biopharmaceutics modelling (PBBM). Two dissolution methods, two-stage (gastrointestinal transfer simulation) and single-phase (biorelevant media), were evaluated under fasted and fed conditions. Clinical BE studies revealed non-BE for formulation A (fasted, N = 39) but compliance for formulation B (fasted/fed, N = 40). Two-stage dissolution highlighted supersaturation dynamics, with fed-state media (FeSSIF-V2) enhancing solubility by >10-fold compared to fasted conditions. Although this method is biopredictive, its complexity limits its practicality. Single-phase dissolution using biorelevant media balances discriminative power and operational feasibility for quality control. Permeability studies identified the active metabolite abiraterone as the absorption driver (apparent permeability coefficient: 1.55 × 10−5 vs. 8.91 × 10−⁶ cm/s for ABA). PBBM integrating hydrolysis kinetics, food effects, and first-pass metabolism predicted clinical pharmacokinetics with a prediction error of <20 %. Virtual BE trials defined a dissolution “safe space” for bioequivalence under both fasted/fed states. This study demonstrates that combining biorelevant dissolution with mechanistic modelling mitigates BE risks for high-variability prodrugs. This single-phase approach offers a scalable and physiologically aligned strategy for guiding the generic development of complex formulations with food-dependent absorption variability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


