手性环己烷连接双咪唑啉的合成。
IF 2.1
4区 化学
Q2 CHEMISTRY, ORGANIC
Beilstein Journal of Organic Chemistry
Pub Date : 2025-09-04
eCollection Date: 2025-01-01
DOI:10.3762/bjoc.21.140
引用次数: 0
摘要
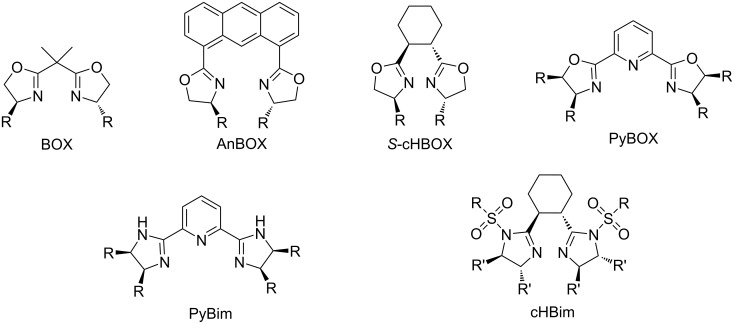
手性双恶唑啉和双咪唑啉都是金属催化不对称有机转化的有效手性配体。以旋光性环己烷-1,2-二羧酸和1,2-二苯基乙烷-1,2-二胺为原料,经1,2-二苯基乙烷-1,2-二胺单磺化,n -磺化的1,2-二苯基乙烷-1,2-二胺和环己烷-1,2-二羧酸缩合,最后用原位生成的Hendrickson试剂环化制备手性环己烷-双咪唑啉。本文章由计算机程序翻译,如有差异,请以英文原文为准。
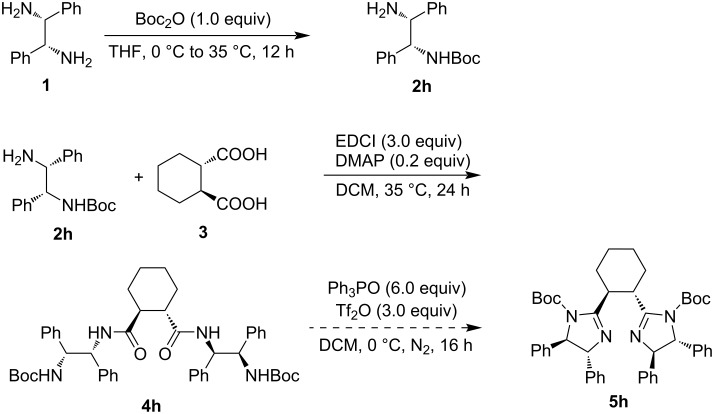
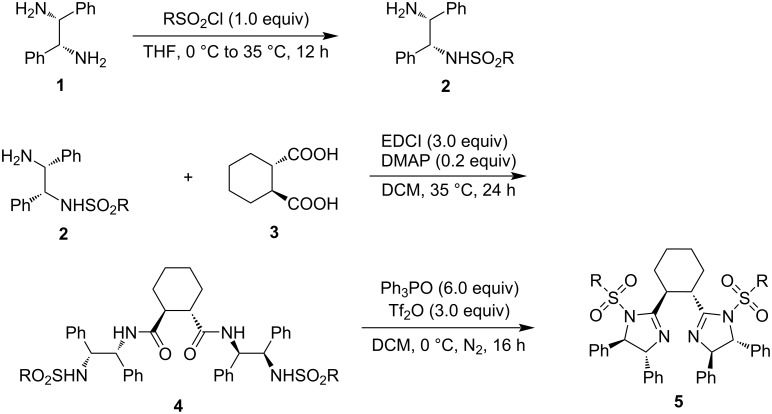
Synthesis of chiral cyclohexane-linked bisimidazolines.
Both chiral bisoxazolines and bisimidazolines are efficient chiral ligands in metal-catalyzed asymmetric organic transformations. Chiral cyclohexane-linked bisimidazolines were prepared from optically active cyclohexane-1,2-dicarboxylic acid and 1,2-diphenylethane-1,2-diamines via the monosulfonylation of 1,2-diphenylethane-1,2-diamines, condensation of N-sulfonylated 1,2-diphenylethane-1,2-diamines and cyclohexane-1,2-dicarboxylic acid, and the final cyclization with the in situ generated Hendrickson reagent.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


