羰基化合物的氨基和n -乙基衍生物中的互变异构、Z/E异构和h键
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文主要分析了近二十年来在N和O原子间质子转移、CC或CN键旋转异构和特定异构体中氢键异构的两类化合物。第一类是胺酮,作为杂环的合成前体,或在结构上作为推拉式乙烯吸引了越来越多的兴趣。这减少了双键周围的旋转障碍,增加了相邻单键周围的旋转障碍。另一类具有互变异构、构象平衡和旋转平衡以及氢键的化合物是在CC键上具有芳香n -杂环的酮醇或二酮醇,这类化合物主要由我们的课题组研究。综述了近年来在E/Z异构、互变异构、h键等方面的研究进展,以及这些现象之间的关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
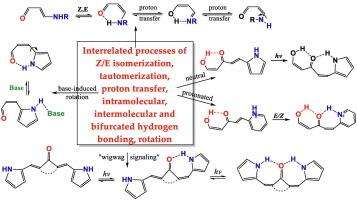
Tautomerism, Z/E isomerization, and H-bonding in the amino and N-hetaryl derivatives of carbonyl compounds
In the present review, two types of compounds capable of tautomerization with proton transfer between N and O atoms, isomerization by rotation about the C![]() C or C
C or C![]() N bonds, and hydrogen bonding in specific isomers are analyzed, focusing on the last two decades. The first type is enaminones attracting a growing interest as synthetic precursors of heterocycles, or, structurally, as push-pull ethylenes. This reduces the barrier to rotation around the double bond, and increases the barrier to rotation around the adjacent single bond. Another type of compounds also capable of tautomerism, conformational and rotational equilibrium, and the hydrogen bonding, is keto- or diketoenols with aromatic N-heterocycles at the C
N bonds, and hydrogen bonding in specific isomers are analyzed, focusing on the last two decades. The first type is enaminones attracting a growing interest as synthetic precursors of heterocycles, or, structurally, as push-pull ethylenes. This reduces the barrier to rotation around the double bond, and increases the barrier to rotation around the adjacent single bond. Another type of compounds also capable of tautomerism, conformational and rotational equilibrium, and the hydrogen bonding, is keto- or diketoenols with aromatic N-heterocycles at the C![]() C bond which have been investigated mainly by our research groups. Overall, the review summarizes recent advances in studying E/Z isomerization, tautomerism, H-bonding in enaminones and ketoenols, and relationship between these phenomena.
C bond which have been investigated mainly by our research groups. Overall, the review summarizes recent advances in studying E/Z isomerization, tautomerism, H-bonding in enaminones and ketoenols, and relationship between these phenomena.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


