NiCl2/NaBH4体系对n -烯丙氧基羰基的脱保护
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们开发了一种利用NiCl₂/NaBH₄体系对n -烯丙基羰基(Alloc)进行脱保护的新方法,以提供收率良好至优异的母氨基化合物。值得注意的是,该实用方案与多种N-alloc保护胺具有良好的兼容性,这些保护胺具有不同的官能团,这些官能团来自多取代苯胺、杂芳烃和脂肪胺,甚至一些仲胺。并对反应机理进行了论证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
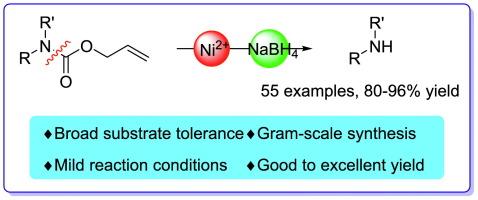
Deprotection of N-Allyloxycarbonyl groups using the NiCl2/NaBH4 system
Herein, we developed a new method for the deprotection of N-allyloxycarbonyl (Alloc) using a NiCl₂/NaBH₄ system to provide the parent amino compounds with good to excellent yields. Notably, this practical protocol exhibits excellent compatibility with various N-alloc protected amines with different functional groups that derived from multi-substituted anilines, heteroaromatic and aliphatic amines or even some secondary amines. The mechanism of the reaction is also demonstrated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


