选择性实时二硫还原与同时荧光标记的人源抗可卡因单抗
IF 2.2
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
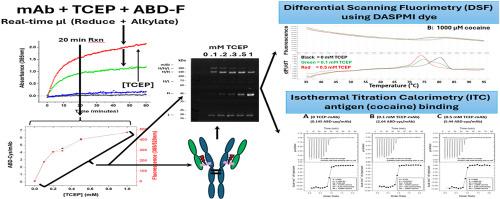
设计并表征了一种快速筛选和开发易于和有限部分还原,同时具有荧光标记的人源抗可卡因单抗的程序。该方法采用一种标准的可溶性蛋白质还原剂三(2-羧乙基)膦(TCEP)与一种无需去除还原剂即可使用的烷基化剂配对,生成半胱氨酸的荧光加合物。该反应也可以通过纳米光度计的吸光度来监测,允许使用小单抗量和体积快速,简单地筛选还原和烷基化条件。还原的程度很容易控制,并且产生的结果类似于单独标记反应后的有限还原,如最近报道使用固定化TCEP和活性较低的磷化氢还原剂,三苯基磷化氢-3,3 ',3″-三磺酸(TPPTS)。单抗的整体结构没有受到干扰,单抗Fab部分的热稳定性(大多数选择性二硫还原发生的地方)仅轻微下降。等温滴定量热法表明,抗原(可卡因)的结合和结合热力学没有改变。然而,差示扫描荧光法显示,在还原标记到5.44修饰的cys/mAb水平后,可卡因结合Fab结构域的热稳定性显著降低,这表明轻重链二硫键对可卡因诱导的mAb Fab热稳定性的重要性。本工作中描述的方法应该有助于单克隆抗体和其他蛋白质中选择性还原和标记二硫化物,帮助分配特定蛋白质二硫化物键的结构和功能重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Selective real-time disulfide reduction with simultaneous fluorescent labeling of a humanized anti-cocaine mAb
A procedure for rapid screening and development of facile and limited partial reduction, with simultaneous fluorescent labeling, of a humanized anti-cocaine mAb was devised and characterized. This employed a standard soluble protein reductant, tris (2-carboxyethyl) phosphine (TCEP), paired with an alkylating agent that can be used without removal of the reductant, yielding a fluorescent adduct of the generated cysteine. This reaction can also be monitored by absorbance in a nanophotometer, allowing for rapid, simple screening of reduction and alkylation conditions using small mAb amounts and volumes. The degree of reduction is readily controllable, and yields results similar to limited reductions followed by separate labeling reactions, as recently reported using immobilized TCEP, and a less reactive phosphine reductant, triphenylphosphine-3,3′,3″-trisulfonic acid (TPPTS). The overall structure of the mAb is not perturbed, and the thermal stability of the Fab portion of the mAb, where most selective disulfide reductions occur, is only minimally decreased. The antigen (cocaine) binding and binding thermodynamics are not changed, as demonstrated by isothermal titration calorimetry. However, differential scanning fluorimetry demonstrated that the thermal stabilization of the Fab domain by cocaine binding is dramatically decreased after reductive labeling to a level of 5.44 modified cys/mAb, suggesting the importance of light-heavy chain disulfide bonds for the cocaine-induced thermal stabilization of the mAb Fab. Methods described in this work should aid in the selective reduction and labeling of disulfides in mAbs and other proteins, assisting the assignment of structural and functional importance to specific protein disulfide bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


