代谢工程和代谢组学分析蓝藻提高琥珀酸盐生产
IF 4.5
2区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
Algal Research-Biomass Biofuels and Bioproducts
Pub Date : 2025-09-01
DOI:10.1016/j.algal.2025.104286
引用次数: 0
摘要
琥珀酸酯是一种多用途的四碳二羧酸,在合成工业化学品、药品和可生物降解聚合物方面起着关键作用。传统的生产方法受到密集的能源需求、复杂的程序和对不可再生化石资源的依赖的限制,阻碍了可持续制造。本研究通过将代谢工程与非靶向代谢组学相结合,在快速生长的蓝细菌长聚球菌PCC 11801中引入了一个强大的可再生生产平台。具体来说,我们在原生蓝藻启动子下设计了异源表达glyoxylate cycle酶、异柠檬酸裂解酶(ICL)和苹果酸合成酶(MS),以避免在TCA循环中脱羧过程中通常遇到的碳损失。同时,利用CRISPR-Cpf1基因组编辑敲除琥珀酸脱氢酶(SDH),该酶通常将琥珀酸转化为富马酸,从而加强琥珀酸的积累。在优化培养条件下,在富集的5× BG11培养基上进行高密度培养,在低光强和提高CO₂的条件下,细胞外琥珀酸滴度可达350 mg/L。综合代谢组学分析显示,琥珀酸盐产量的增加与中心碳代谢中的广泛重编程有关,其特征是糖酵解通量的增加、TCA循环中间体的积累、氨基酸谱和氧化还原平衡的显著变化,但也施加了代谢应激。这些发现强调了将基因工程与先进的代谢组学分析和培养条件优化相结合的有效性和前景,以促进琥珀酸盐和其他增值化学品的可持续光合生产。本文章由计算机程序翻译,如有差异,请以英文原文为准。
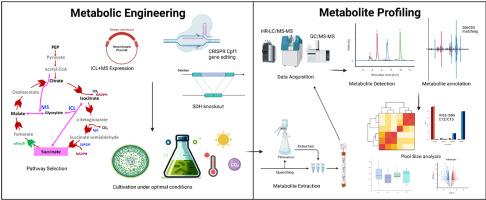
Metabolic engineering and metabolomics based profiling of cyanobacteria for enhanced succinate production
Succinate, a versatile four‑carbon dicarboxylic acid, is pivotal in synthesizing industrial chemicals, pharmaceuticals, and biodegradable polymers. Traditional production methods are constrained by intensive energy requirements, complex procedures, and reliance on non-renewable fossil resources, impeding sustainable manufacturing. This study introduces a robust and renewable production platform by integrating metabolic engineering with untargeted metabolomics in the fast-growing cyanobacterium Synechococcus elongatus PCC 11801. Specifically, we engineered heterologous expression of glyoxylate cycle enzymes, isocitrate lyase (ICL) and malate synthase (MS), under native cyanobacterial promoters to circumvent carbon loss typically encountered during decarboxylation in the TCA cycle. Concurrently, CRISPR-Cpf1 genome editing was utilized to knock out succinate dehydrogenase (SDH), which ordinarily diverts succinate toward fumarate, thus reinforcing succinate accumulation. High cell density cultivation in enriched 5× BG11 medium under optimized culture conditions with low light intensity and elevated CO₂ gave up to 350 mg/L extracellular succinate titer. Comprehensive metabolome profiling revealed that increased succinate production was associated with extensive reprogramming within central carbon metabolism, marked by enhanced glycolytic throughput, accumulation of TCA cycle intermediates, and pronounced changes in amino acid profiles and redox balance, but also imposed metabolic stress. These findings emphasize the effectiveness and promise of integrating genetic engineering with advanced metabolomic profiling and optimization of cultivation conditions to facilitate the sustainable photosynthetic production of succinate and other value-added chemicals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Algal Research-Biomass Biofuels and Bioproducts
BIOTECHNOLOGY & APPLIED MICROBIOLOGY-
CiteScore
9.40
自引率
7.80%
发文量
332
期刊介绍:
Algal Research is an international phycology journal covering all areas of emerging technologies in algae biology, biomass production, cultivation, harvesting, extraction, bioproducts, biorefinery, engineering, and econometrics. Algae is defined to include cyanobacteria, microalgae, and protists and symbionts of interest in biotechnology. The journal publishes original research and reviews for the following scope: algal biology, including but not exclusive to: phylogeny, biodiversity, molecular traits, metabolic regulation, and genetic engineering, algal cultivation, e.g. phototrophic systems, heterotrophic systems, and mixotrophic systems, algal harvesting and extraction systems, biotechnology to convert algal biomass and components into biofuels and bioproducts, e.g., nutraceuticals, pharmaceuticals, animal feed, plastics, etc. algal products and their economic assessment
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


