基于杂环芳酰胺的P-gp抑制剂在乳腺癌自噬抗耐药中的辅助调节作用的发现
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
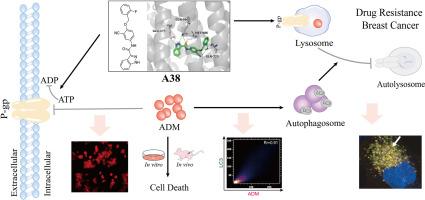
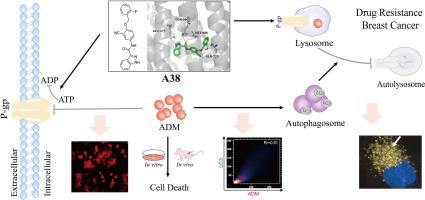
p -糖蛋白(P-gp)在化疗过程中多药耐药(MDR)的发生中起着至关重要的作用,它介导ADM溶酶体的固存,导致耐药的发生。溶酶体与自噬体融合产生自溶酶体,其内容物被降解,从而实现细胞自身的代谢需要和细胞器的更新。本研究设计并合成了一系列N-(3-氰基-4-(烷氧基)苯基)杂环芳香酰胺衍生物(A1-A48)作为新型P-gp抑制剂。其中,化合物A38具有较低的细胞毒性和良好的逆转活性,被认为是最有前途的P-gp抑制剂。P-gp atp酶测定、MOE和CETSA显示A38与P-gp相互作用。通过ADM积累试验等研究证实化合物A38抑制P-gp功能。进一步研究发现,A38联合ADM可显著促进耐药乳腺癌细胞凋亡,显著抑制耐药乳腺癌细胞的增殖、迁移和侵袭能力。mCherry-GFP-LC3B斑点形成实验证实P-gp抑制剂A38阻断自噬通量的形成,ADM联合A38导致自噬小体积聚,从而诱导耐药乳腺癌细胞死亡。在体内和体外肿瘤模型实验中,ADM联合P-gp抑制剂A38的抗肿瘤活性优于tariquidar。化合物A38的有效性及其在调节自噬中的作用为P-gp抑制剂的开发和耐药乳腺癌的临床治疗提供了新的参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of a heterocyclic aromatic amide based P-gp inhibitor as coadjutant regulating autophagy against drug resistance in breast cancer
P-glycoprotein (P-gp) plays a vital role in the development of multidrug resistance (MDR) during chemotherapy, which mediated lysosomal sequestration of ADM, leading to the development of drug resistance. Lysosomes fuse with autophagosomes to generate autolysosomes in which contents are degraded, thereby achieving the metabolic needs of the cell itself and the renewal of organelles. In this work, a series of N-(3-cyano-4-(alkoxy)phenyl)heterocyclic aromatic amide derivatives (A1-A48) were designed and synthesized as novel P-gp inhibitors. Among them, compound A38 showed low cytotoxicity and excellent reversal activity, which was considered the most promising P-gp inhibitor. P-gp ATPase assay, MOE and CETSA revealed that A38 interacts with P-gp. ADM accumulation assay and other studies were conducted to verify that compound A38 inhibits P-gp function. Further investigation indicated that A38 in combination with ADM significantly promoted apoptosis and markedly suppressed the proliferation, migration and invasion ability in drug resistant breast cancer. mCherry-GFP-LC3B puncta formation assay verified that P-gp inhibitor A38 blocks the formation of autophagic flux and ADM combined with A38 results in the accumulation of autophagosomes, thereby inducing drug-resistant breast cancer cell death. In in vivo and in vitro tumor model experiments, the antitumor activity of ADM combined with P-gp inhibitor A38 was superior to that of tariquidar. The effectiveness of compound A38 and its role in regulating autophagy provided a new reference for the development of P-gp inhibitors and the clinical treatment of drug-resistant breast cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


