剂量组学指导下的深度学习用于肺癌放射性食管炎预测:通过多分支融合辅助学习确定最佳感兴趣区域。
IF 5.3
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
背景:在基于放射组学的预测模型中,准确描绘感兴趣区域(roi)对于特征提取和选择至关重要。目的:为了建立剂量组学和深度学习(DL)相结合的预测肺癌放疗患者 ≥ 2级放射性食管炎(RE)的模型,我们提出了一种基于放射剂量分布(RDD)图像的多任务辅助学习方法来定义准确客观的roi。材料和方法:回顾性收集1号医院(2020年1月至2022年12月)接受放疗的肺癌患者进行模型开发。回顾性地从医院2(2021年1月和2022年12月)和医院3(2022年1月和2023年12月)获得两个外部验证集。通过将剂量组学特征与高维深度学习特征相结合,提出了一种基于多任务辅助学习的剂量组学引导深度学习(DGD)网络,用于精确、客观地定义roi。结果:本研究纳入了来自三家医院的488例患者,其中训练集235例,内部验证集101例,外部验证集1 57例,外部验证集2 95例。以剂量组学为指导的ResNet34结合对比学习和辅助分割模块,在内部验证集、外部验证集1和外部验证集2中分别获得了0.88 [95% CI: 0.76-0.95]、0.82 [95% CI: 0.65-0.96]、0.83 [95% CI: 0.74-0.92]的最佳auc。结论:提出的DGD模型利用多任务辅助学习自动定义roi,有效预测肺癌放疗患者的RE。本文章由计算机程序翻译,如有差异,请以英文原文为准。
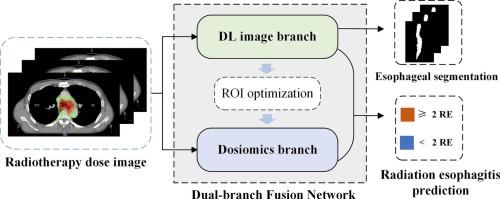
Dosiomics-guided deep learning for radiation esophagitis prediction in lung cancer: optimal region of interest definition via multi-branch fusion auxiliary learning
Background
Accurate delineation of regions of interest (ROIs) is critical for feature extraction and selection in radiomics-based prediction models.
Purpose
To develop a combined dosiomics and deep learning (DL) model for predicting grade ≥ 2 radiation esophagitis (RE) in lung cancer patients undergoing radiotherapy, we propose a multi-task auxiliary learning approach to define accurate and objective ROIs based on radiation dose distribution (RDD) images.
Materials and Methods
Lung cancer patients who underwent radiotherapy were gathered retrospectively from hospital 1 (January 2020 and December 2022) for model development. Two external validation sets were obtained retrospectively from hospital 2 (January 2021 and December 2022) and hospital 3 (January 2022 and December 2023), respectively. A dosiomics-guided deep learning (DGD) network using multi-task auxiliary learning to define accurate and objective ROIs was introduced by integrating dosiomic features with high-dimensional DL features for RE prediction.
Results
This study enrolled 488 patients from three hospitals: 235 in the training set, 101 in the internal validation set, 57 in the external validation set 1 and 95 in the external validation set 2, respectively. The dosiomics −guided ResNet34 combined with contrastive learning and auxiliary segmentation module achieved the best AUCs of 0.88 [95% CI: 0.76–0.95], 0.82 [95% CI: 0.65–0.96], 0.83 [95% CI: 0.74–0.92] in the internal validation set, external validation set 1, and external validation set 2, respectively.
Conclusion
The proposed DGD model leverages multi-task auxiliary learning to automatically define ROIs and effectively predict RE in lung cancer patients undergoing radiotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Radiotherapy and Oncology
医学-核医学
CiteScore
10.30
自引率
10.50%
发文量
2445
审稿时长
45 days
期刊介绍:
Radiotherapy and Oncology publishes papers describing original research as well as review articles. It covers areas of interest relating to radiation oncology. This includes: clinical radiotherapy, combined modality treatment, translational studies, epidemiological outcomes, imaging, dosimetry, and radiation therapy planning, experimental work in radiobiology, chemobiology, hyperthermia and tumour biology, as well as data science in radiation oncology and physics aspects relevant to oncology.Papers on more general aspects of interest to the radiation oncologist including chemotherapy, surgery and immunology are also published.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


